Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Intensive Salvage Chemotherapy and Hypomethylating Agents
3. New Approaches for the Treatment of Relapse of AML in Older Patients
3.1. FLT3 Inhibitors
3.2. IDH1 Inhibitors
4. Conclusions
Funding
Conflicts of Interest
References
- National Cancer Institute. Seer Cancer Statistics Factsheets: Acute Myeloid Leukemia; National Cancer Institute: Bethesda, MD, USA, 2018. Available online: http://seer.Cancer.Gov/statfacts/html/amyl.Html (accessed on 20 May 2018).
- Juliusson, G.; Lazarevic, V.; Hörstedt, A.S.; Hagberg, O.; Höglund, M.; Swedish Acute Leukemia Registry Group. Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood 2012, 119, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.A.; Pratz, K.W. Acute myeloid leukemia in the elderly: Therapeutic options and choice. Leuk. Lymphoma 2018, 59, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K. Treatment of Older Patients with Newly Diagnosed AML Unfit for Traditional Therapy. Clin. Lymphoma Myeloma Leuk. 2018, 18, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Podoltsev, N.A.; Stahl, M.; Zeidan, A.M.; Gore, S.D. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev. 2017, 31, 43–62. [Google Scholar] [CrossRef]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2018. [Google Scholar] [CrossRef]
- Bell, J.A.; Galaznik, A.; Huelin, R.; Stokes, M.; Guo, Y.; Fram, R.J.; Faller, D.V. Effectiveness and Safety of Therapeutic Regimens for Elderly Patients with Acute Myeloid Leukemia: A Systematic Literature Review. Clin. Lymphoma Myeloma Leuk. 2018, 18, e303–e314. [Google Scholar] [CrossRef]
- Michaelis, L.C.; Klepin, H.D.; Walter, R.B. Advancements in the management of medically less-fit and older adults with newly diagnosed acute myeloid leukemia. Expert Opin. Pharmacother. 2018, 19, 865–882. [Google Scholar] [CrossRef]
- Dombret, H.; Itzykson, R. How and when to decide between epigenetic therapy and chemotherapy in patients with AML. Hemato. Am. Soc. Hematol. Educ. Program. 2017, 8, 45–53. [Google Scholar]
- Gardin, C.; Dombret, H. Hypomethylating Agents as a Therapy for AML. Curr. Hematol. Malig. Rep. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, P.F.; Zhou, J.D.; Yao, D.M.; Ma, J.C.; Wen, X.M.; Zhang, Z.H.; Lian, X.Y.; Xu, Z.J.; Qian, J.; Lin, J. Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Oncotarget 2017, 8, 41498–41507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenti, E.; Papayannidis, C.; Marconi, G.; Parisi, S.; Simonetti, G.; Paolini, S.; Sartor, C.; Ottaviani, E.; Testoni, N.; Martinelli, G. Efficacy of Azacitidine in the treatment of adult patients aged 65 years or older with AML. Expert Opin. Pharmacother. 2016, 17, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, C.D.; Estey, E.; Pleyer, L.; Schuh, A.C.; Stein, E.M.; Tallman, M.S.; Wei, A. Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018, 32, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F. Is complete remission key in elderly patients with AML? Lancet Haematol. 2016, 3, e212–e213. [Google Scholar] [CrossRef]
- Ferrara, F. Conventional chemotherapy or hypomethylating agents for older patients with acute myeloid leukaemia? Hematol. Oncol. 2014, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Palmieri, S.; Mele, G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica 2004, 89, 998–1008. [Google Scholar] [PubMed]
- Medeiros, B.C. Is there a standard of care for relapsed AML? Best Pract. Res. Clin. Haematol. 2018, 31, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Sanz, M.Á.; Montesinos, P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AMLpatients: A systematic literature review. Ann. Hematol. 2018, 97, 1115–1153. [Google Scholar] [CrossRef]
- Rashidi, A.; Weisdorf, D.J.; Bejanyan, N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br. J. Haematol. 2018, 181, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Müller-Tidow, C.; Benner, A.; Kieser, M. Relapsed/refractory acute myeloid leukemia: Any progress? Curr. Opin. Oncol. 2017, 29, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Lancet, J.E. Is the overall survival for older adults with AML finally improving? Best Pract. Res. Clin. Haematol. 2018, 31, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Breems, D.A.; Van Putten, W.L.; Huijgens, P.C.; Ossenkoppele, G.J.; Verhoef, G.E.; Verdonck, L.F.; Vellenga, E.; De Greef, G.E.; Jacky, E.; Van der Lelie, J.; et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 2005, 23, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Lipof, J.J.; Loh, K.P.; O’Dwyer, K.; Liesveld, J.L. Allogeneic Hematopoietic Cell Transplantation for Older Adults with Acute Myeloid Leukemia. Cancers (Basel) 2018, 10, 179. [Google Scholar] [CrossRef]
- Levin-Epstein, R.; Oliai, C.; Schiller, G. Allogeneic Hematopoietic Stem Cell Transplantation for Older Patients with Acute Myeloid Leukemia. Curr. Treat. Opt. Oncol. 2018, 19, 63. [Google Scholar] [CrossRef]
- Veltri, L.; Rezvani, K.; Oran, B.; Mehta, R.; Rondon, G.; Kebriaei, P.; Popat, U.; Nieto, Y.; Hosing, C.; Qazilbash, M.; et al. Allotransplants for Patients 65 Years or Older with High-Risk Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018. [Google Scholar] [CrossRef]
- Hecker, J.; Miller, I.; Götze, K.S.; Verbeek, M. Bridging Strategies to Allogeneic Transplant for Older AML Patients. Cancers (Basel) 2018, 10, 232. [Google Scholar] [CrossRef]
- Stahl, M.; DeVeaux, M.; Montesinos, P.; Itzykson, R.; Ritchie, E.K.; Sekeres, M.A.; Barnard, J.D.; Podoltsev, N.A.; Brunner, A.M.; Komrokji, R.S.; et al. Hypomethylating agents in relapsed and refractory AML: Outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 923–932. [Google Scholar] [CrossRef]
- Laurino, M.; Lessi, F.; Candoni, A.; Lazzarotto, D.; Papayannidis, C.; Gottardi, M.; Vitaglian, O.; Reda, G.; Riva, M.; Crisà, E.; et al. Hypometilating agents (HMA) as salvage therapy in relapsed or refractory AML: An Italian 9-center retrospective study. HemaSphere 2018, 2 (Suppl. 1), PF253. [Google Scholar]
- Craddock, C.; Labopin, M.; Robin, M.; Finke, J.; Chevallier, P.; Yakoub-Agha, I.; Bourhis, J.H.; Sengelov, H.; Blaise, D.; Luft, T.; et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2016, 101, 879–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbour, E.J.; Garcia-Manero, G.; Strati, P.; Mishra, A.; Al Ali, N.H.; Padron, E.; Lancet, J.; Kadia, T.; Daver, N.; O’Brien, S.; et al. Outcome of patients with low risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: A report on behalf of the MDS Clinical Research Consortium. Cancer 2015, 121, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Prébet, T.; Gore, S.D.; Esterni, B.; Gardin, C.; Itzykson, R.; Thepot, S.; Dreyfus, F.; Rauzy, O.B.; Recher, C.; Adès, L.; et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J. Clin. Oncol. 2011, 29, 3322–3327. [Google Scholar] [CrossRef]
- Carraway, H.E. Treatment options for patients with myelodysplastic syndromes after hypomethylating agent failure. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldoss, I.; Yang, D.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Mei, M.; Salhotra, A.; Khaled, S.; Nakamura, R.; et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 2018, 103, e404–e407. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Burd, A.; Ross, L.; Levine, L.R.; Shoben, A.; Byrd, J.C.; Druker, B.J.; Stefanos, M.; Marcus, S.; Rosenberg, L.; Vittorio, M.; et al. Initial Report of the Beat AML Umbrella Study for Previously Untreated AML: Evidence of Feasibility and Early Success in Molecularly Driven Phase 1 and 2 Studies. Blood 2018, 132 (Suppl. 1), Abs559. [Google Scholar]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Bullinger, L.; Dohner, K.; Dohner, H. Genomics of acute myeloid leukemia diagnosis and pathways. J. Clin. Oncol. 2017, 35, 934–946. [Google Scholar] [CrossRef]
- Larrosa-Garcia, M.; Maria RBaer, M.R. FLT3 inhibitors in acute myeloid leukemia: Current status and future directions. Mol. Cancer Ther. 2017, 16, 991–1001. [Google Scholar] [CrossRef]
- Eisfeld, A.K.; Kohlschmidt, J.; Mrózek, K.; Blachly, J.S.; Walker, C.J.; Nicolet, D.; Orwick, S.; Maharry, S.E.; Carroll, A.J.; Stone, R.M.; et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: An analysis of Alliance studies. Leukemia 2018, 32, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Hou, H.A.; Tang, J.L.; Liu, C.Y.; Lin, C.C.; Chou, W.C.; Tseng, M.H.; Chiang, Y.C.; Kuo, Y.Y.; Liu, M.C.; et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia 2016, 30, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: A multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017, 18, 1061–1075. [Google Scholar] [CrossRef]
- Cortes, J.; Perl, A.E.; Döhner, H.; Kantarjian, H.; Martinelli, G.; Kovacsovics, T.; Rousselot, P.; Steffen, B.; Dombret, H.; Estey, E. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018, 19, 889–903. [Google Scholar] [CrossRef]
- Cortes, J.; Khaled, S.K.; Martielli, G.; Perl, A.E.; Ganguly, S.; Russell, N.H.; Kramer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Efficacy and Safety of Single-Agent Quizartinib (Q), a Potent and Selective FLT3 Inhibitor (FLT3i), in Patients (pts) with FLT3-Internal Tandem Duplication (FLT3-ITD)–Mutated Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) Enrolled in the Global, Phase 3, Randomized Controlled Quantum-R Trial. Blood 2018, 132 (Suppl. 1), Abst563. [Google Scholar]
- Montalban-Bravo, G.; DiNardo, C.D. The role of IDH mutations in acute myeloid leukemia. Future Oncol. 2018, 14, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Fathi, A.T.; DiNardo, C.D.; Pollyea, D.A.; Chan, S.M.; Swords, R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia 2017, 31, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Clark, O.; Yen, K.; Mellinghoff, I.K. Molecular Pathways: Isocitrate Dehydrogenase Mutations in Cancer. Clin. Cancer Res. 2016, 22, 1837–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNardo, C.D.; Stein, E.M. SOHO State of the Art Update and Next Questions: IDH Therapeutic Targeting in AML. Clin. Lymphoma Myeloma Leuk. 2018, 18, 769–772. [Google Scholar] [CrossRef]
- Abou Dalle, I.; DiNardo, C.D. The role of enasidenib in the treatment of mutant IDH2 acute myeloid leukemia. Ther. Adv. Hematol. 2018, 9, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Buege, M.J.; DiPippo, A.J.; DiNardo, C.D. Evolving Treatment Strategies for Elderly Leukemia Patients with IDH Mutations. Cancers (Basel) 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Nassereddine, S.; Lap, C.J.; Tabbara, I.A. Evaluating ivosidenib for the treatment of relapsed/refractory AML: Design, development, and place in therapy. Oncol. Targets Ther. 2018, 12, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Pollyea, D.A.; Stone, R.M.; Altman, J.K.; Roboz, G.J.; Patel, M.R.; Collins, R.; Flinn, I.W.; et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 2018. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.T.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Gale, R.; Linch, D.; Huntly, B.J.P.; Papaemmanuil, E.; Vyas, P.; FRCPath, M.F.; Burnett, A.K.; Russell, N.H. Outcomes of Relapsed/Refractory Patients with IDH1/2 Mutated AML Treated with Non-Targeted Therapy: Results from the NCRI AML Trials. Blood 2018, 132 (Suppl. 1), Abst664. [Google Scholar]
- Norsworthy, K.J.; Mulkey, F.; Ward, A.F.; Przepiorka, D.; Deisseroth, A.B.; Farrell, A.T. Incidence of Differentiation Syndrome with Ivosidenib (IVO) and Enasidenib (ENA) for Treatment of Patients with Relapsed or Refractory (R/R) Isocitrate Dehydrogenase (IDH)1- or IDH2-Mutated Acute Myeloid Leukemia (AML): A Systematic Analysis by the U.S. Food and Drug Administration (FDA). Blood 2018, 132 (Suppl. 1), Abst288. [Google Scholar]
- Sanz, M.A.; Montesinos, P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood 2014, 123, 2777–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, A.T.; DiNardo, C.D.; Kline, I.; Kenvin, L.; Gupta, I.; Attar, E.C.; Stein, E.M.; de Botton, S.; AG221-C-001 Study Investigators. Differentiation Syndrome Associated with Enasidenib, a Selective Inhibitor of Mutant Isocitrate Dehydrogenase 2: Analysis of a Phase 1/2 Study. JAMA Oncol. 2018, 4, 1106–1110. [Google Scholar] [CrossRef]
- Prassek, V.V.; Rothenberg-Thurley, M.; Sauerland, M.C.; Herold, T.; Janke, H.; Ksienzyk, B.; Konstandin, N.P.; Goerlich, D.; Krug, U.; Faldum, A.; et al. Genetics of acute myeloid leukemia in the elderly: Mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica 2018, 103, 1853–1861. [Google Scholar] [CrossRef]
- Döhner, H.; Dolnik, A.; Tang, L.; Seymour, J.F.; Minden, M.D.; Stone, R.M.; Del Castillo, T.B.; Al-Ali, H.K.; Santini, V.; Vyas, P.; et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia 2018, 32, 2546–2557. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Martelli, M.P. Dactinomycin in NPM1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Bigliardi, S.; Quadrelli, C.; Morselli, M.; Potenza, L.; Paolini, A.; Colaci, E.; Barozzi, P.; Zucchini, P.; Riva, G.; et al. All-trans retinoic acid (ATRA) in non-promyelocytic acute myeloid leukemia (AML): Results of combination of ATRA with low-dose Ara-C in three elderly patients with NPM1-mutated AML unfit for intensive chemotherapy and review of the literature. Clin. Case Rep. 2016, 4, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Morabito, F.; Latagliata, R.; Martino, B.; Annunziata, M.; Oliva, E.; Schiavone, E.M.; Pollio, F.; Palmieri, S.; Gianfaldoni, G.; et al. Aggressive salvage treatment is not appropriate for the majority of elderly patients with acute myeloid leukemia relapsed from first complete remission. Haematologica 2001, 86, 814–820. [Google Scholar] [PubMed]
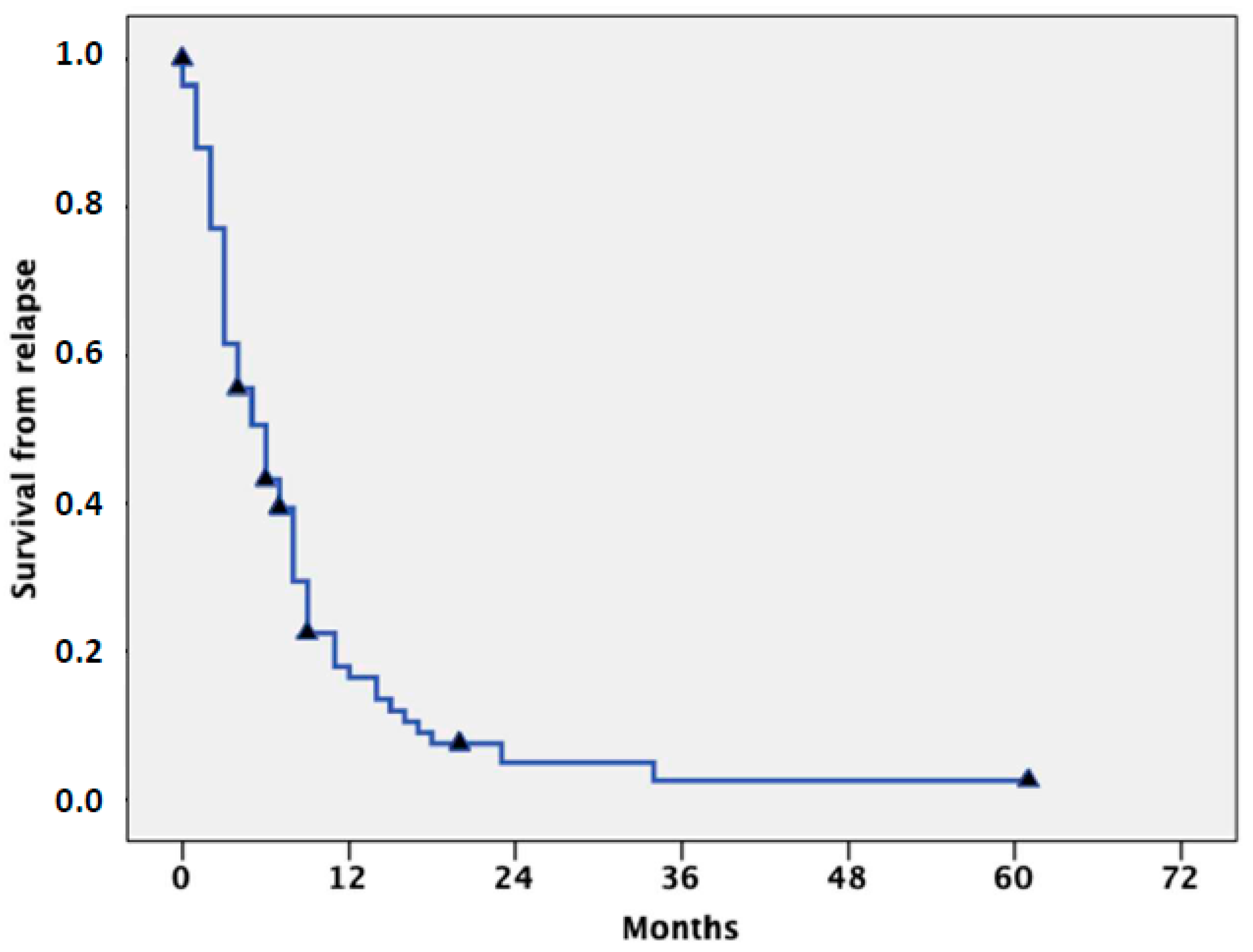
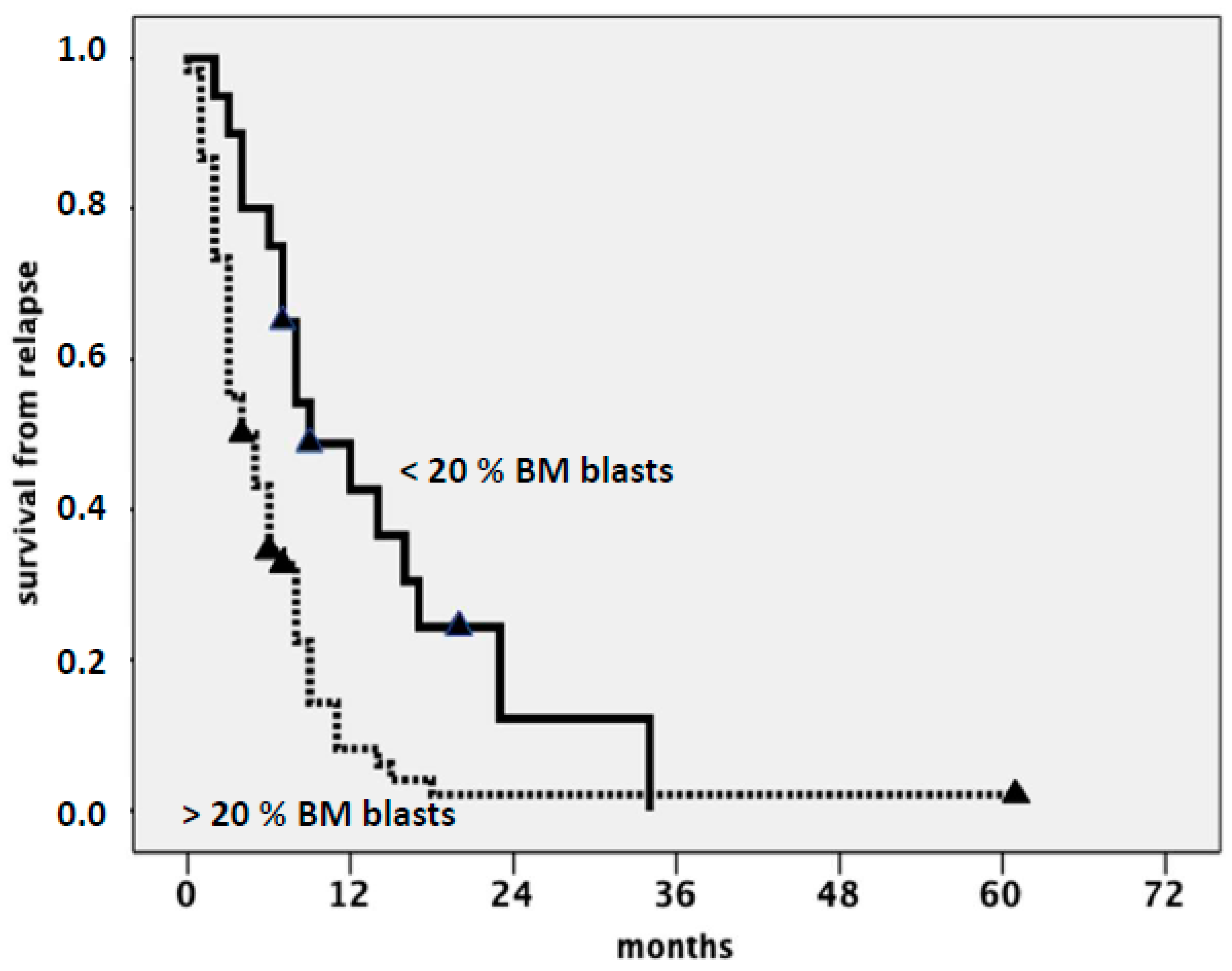


| Drug [Ref.] | Target | ORR (CR + CRi) | Median Survival (Months) | Other Benefits |
|---|---|---|---|---|
| Gilteritinib [43] | FLT3 | 21% | 4.6 | Sustained transfusion independence (31%) |
| Quizartinib [45] | FLT3 | 48% | 6.2 | Benefit across subgroups, including varying allelic ratio, prior HCT, AML risk score, and response to prior therapy |
| Ivosidenib [54] | IDH1 | 33% | 8.8 | Sustained transfusion independence (35%) |
| Enasidenib [53] | IDH2 | 23% | 8.2 | Sustained transfusion independence (34%) |
| AZA or DAC [30] | DNA methylation | 16% | 6.7 | Hematological improvement (8%) |
| AZA/DAC + Venetoclax [36] | DNA methylation BCL2 regulation | 51% | 6.5 | 10% morphological leukemia free state |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, F.; Lessi, F.; Vitagliano, O.; Birkenghi, E.; Rossi, G. Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers 2019, 11, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11020224
Ferrara F, Lessi F, Vitagliano O, Birkenghi E, Rossi G. Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers. 2019; 11(2):224. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11020224
Chicago/Turabian StyleFerrara, Felicetto, Federica Lessi, Orsola Vitagliano, Erika Birkenghi, and Giuseppe Rossi. 2019. "Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia" Cancers 11, no. 2: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers11020224




