Consensus Definition and Prediction of Complexity in Transurethral Resection or Bladder Endoscopic Dissection of Bladder Tumours
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
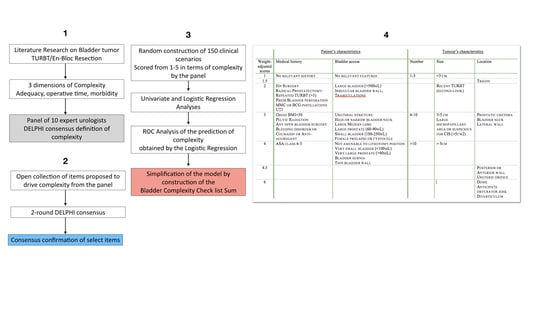
2.1. Step 1: Definition of Complexity
2.2. Step 2: Items That Drive Complexity
2.3. Step 3: Construction, Discrimination and Accuracy of the Bladder Complexity Checklist Sum
2.3.1. Clinical Scenarios
2.3.2. Discrimination and Accuracy
3. Discussion
4. Materials and Methods
4.1. Step 1: Consensus Definition of Complexity
4.2. Step 2: Listing the Items That Drive Complexity
4.2.1. Collection of the Factors Related to Complexity
4.2.2. Delphi Validation
4.3. Step 3: Construction of the Bladder Complexity Checklist
4.3.1. Construction of Clinical Scenarios
4.3.2. Discrimination of Individual Items in the Prediction of Complexity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.F.; Pareek, G.; Mueller-Leonhard, C.; Zhang, Z.; Amin, A.; Mega, A.; Tucci, C.; Golijanin, D.; Gershman, B. The Perioperative Morbidity of Transurethral Resection of Bladder Tumor: Implications for Quality Improvement. Urology 2019, 125, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, B.K.; Miller, D.C.; Taub, D.; Dunn, R.L.; Khuri, S.F.; Henderson, W.G.; Montie, J.E.; Underwood, W.; Wei, J.T., 3rd; Wei, J.T. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006, 106, 1527–1535. [Google Scholar] [CrossRef] [Green Version]
- Cumberbatch, M.G.K.; Foerster, B.; Catto, J.W.F.; Kamat, A.M.; Kassouf, W.; Jubber, I.; Shariat, S.F.; Sylvester, R.J.; Gontero, P. Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 925–933. [Google Scholar] [CrossRef]
- Mariappan, P.; Finney, S.M.; Head, E.; Somani, B.K.; Zachou, A.; Smith, G.; Mishriki, S.F.; N’Dow, J.; Grigor, K.M.; Edinburgh Urological Cancer G. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: Validation across time and place and recommendation for benchmarking. BJU Int. 2012, 109, 1666–1673. [Google Scholar]
- Ghali, F.; Moses, R.A.; Raffin, E.; Hyams, E.S. What factors are associated with unplanned return following transurethral resection of bladder tumor? An analysis of a large single institution’s experience. Scand. J. Urol. 2016, 50, 370–373. [Google Scholar] [CrossRef]
- Mariappan, P.; Zachou, A.; Grigor, K.M.; Edinburgh Uro-Oncology Group. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur. Urol. 2010, 57, 843–849. [Google Scholar] [CrossRef]
- Gan, C.; Patel, A.; Fowler, S.; Catto, J.; Rosario, D.; O’Brien, T. Snapshot of transurethral resection of bladder tumours in the United Kingdom Audit (STUKA). BJU Int. 2013, 112, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.N.; Muddukrishna, S.N. Quality of transurethral resection of bladder tumor procedure influenced a phase III trial comparing the effect of KLH and mitomycin C. Trials 2017, 18, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzypczyk, M.A.; Nyk, L.; Szostek, P.; Szemplinski, S.; Borowka, A.; Dobruch, J. The role of endoscopic bladder tumour assessment in the management of patients subjected to transurethral bladder tumour resection. Eur. J. Cancer Care (Engl.) 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, A.; Pace, G.; Masciovecchio, S.; Saldutto, P.; Galatioto, G.P.; Vicentini, C. Plasmakinetic bipolar versus monopolar transurethral resection of non-muscle invasive bladder cancer: A single center randomized controlled trial. Int. J. Urol. 2013, 20, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Soloway, M.S. The importance of transurethral resection in managing patients with urothelial cancer in the bladder: Proposal for a transurethral resection of bladder tumor checklist. Eur. Urol. 2012, 61, 1199–1203. [Google Scholar] [CrossRef]
- Venkatramani, V.; Panda, A.; Manojkumar, R.; Kekre, N.S. Monopolar versus bipolar transurethral resection of bladder tumors: A single center, parallel arm, randomized, controlled trial. J. Urol. 2014, 191, 1703–1707. [Google Scholar] [CrossRef]
- Wu, Y.P.; Lin, T.T.; Chen, S.H.; Xu, N.; Wei, Y.; Huang, J.B.; Sun, X.L.; Zheng, Q.S.; Xue, X.Y.; Li, X.D. Comparison of the efficacy and feasibility of en bloc transurethral resection of bladder tumor versus conventional transurethral resection of bladder tumor: A meta-analysis. Medicine (Baltimore) 2016, 95, e5372. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Xing, J.C.; Li, W.; Wu, Z.; Chen, B.; Bai, D.Y. A novel transurethral resection technique for superficial bladder tumor: Retrograde en bloc resection. World J. Surg. Oncol. 2017, 15, 125. [Google Scholar] [CrossRef]
- Herkommer, K.; Hofer, C.; Gschwend, J.E.; Kron, M.; Treiber, U. Gender and body mass index as risk factors for bladder perforation during primary transurethral resection of bladder tumors. J. Urol. 2012, 187, 1566–1570. [Google Scholar] [CrossRef]
- Golan, S.; Baniel, J.; Lask, D.; Livne, P.M.; Yossepowitch, O. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair—Clinical characteristics and oncological outcomes. BJU Int. 2011, 107, 1065–1068. [Google Scholar] [CrossRef]
- Carmignani, L.; Picozzi, S.; Stubinski, R.; Casellato, S.; Bozzini, G.; Lunelli, L.; Arena, D. Endoscopic resection of bladder cancer in patients receiving double platelet antiaggregant therapy. Surg. Endosc. 2011, 25, 2281–2287. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, K.; Yang, H.; Xia, D.; Chen, Z. Bipolar Versus Monopolar Transurethral Resection of Nonmuscle-Invasive Bladder Cancer: A Meta-Analysis. J. Endourol. 2016, 30, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Yasunaga, H.; Horiguchi, H.; Matsui, H.; Nishimatsu, H.; Nakagawa, T.; Fushimi, K.; Kattan, M.W.; Homma, Y. Comparison of perioperative outcomes including severe bladder injury between monopolar and bipolar transurethral resection of bladder tumors: A population based comparison. J. Urol. 2014, 192, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Allard, C.B.; Meyer, C.P.; Gandaglia, G.; Chang, S.L.; Chun, F.K.; Gelpi-Hammerschmidt, F.; Hanske, J.; Kibel, A.S.; Preston, M.A.; Trinh, Q.D. The Effect of Resident Involvement on Perioperative Outcomes in Transurethral Urologic Surgeries. J. Surg. Educ. 2015, 72, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Ball, M.W.; Cohen, J.E.; Kates, M.; Pierorazio, P.M.; Allaf, M.E. Morbidity of urologic surgical procedures: An analysis of rates, risk factors, and outcomes. Urology 2015, 85, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Avallone, M.A.; Sack, B.S.; El-Arabi, A.; Charles, D.K.; Herre, W.R.; Radtke, A.C.; Davis, C.M.; See, W.A. Ten-Year Review of Perioperative Complications After Transurethral Resection of Bladder Tumors: Analysis of Monopolar and Plasmakinetic Bipolar Cases. J. Endourol. 2017, 31, 767–773. [Google Scholar] [CrossRef]
- Rambachan, A.; Matulewicz, R.S.; Pilecki, M.; Kim, J.Y.; Kundu, S.D. Predictors of readmission following outpatient urological surgery. J. Urol. 2014, 192, 183–188. [Google Scholar] [CrossRef]
- Matulewicz, R.S.; Pilecki, M.; Rambachan, A.; Kim, J.Y.; Kundu, S.D. Impact of resident involvement on urological surgery outcomes: An analysis of 40,000 patients from the ACS NSQIP database. J. Urol. 2014, 192, 885–890. [Google Scholar] [CrossRef]
- Picozzi, S.; Marenghi, C.; Ricci, C.; Bozzini, G.; Casellato, S.; Carmignani, L. Risks and complications of transurethral resection of bladder tumor among patients taking antiplatelet agents for cardiovascular disease. Surg. Endosc. 2014, 28, 116–121. [Google Scholar] [CrossRef]
- De Nunzio, C.; Franco, G.; Cindolo, L.; Autorino, R.; Cicione, A.; Perdona, S.; Falsaperla, M.; Gacci, M.; Leonardo, C.; Damiano, R.; et al. Transuretral resection of the bladder (TURB): Analysis of complications using a modified Clavien system in an Italian real life cohort. Eur. J. Surg. Oncol. 2014, 40, 90–95. [Google Scholar] [CrossRef]
- Valerio, M.; Cerantola, Y.; Fritschi, U.; Hubner, M.; Iglesias, K.; Legris, A.S.; Lucca, I.; Vlamopoulos, Y.; Vaucher, L.; Jichlinski, P. Comorbidity and nutritional indices as predictors of morbidity after transurethral procedures: A prospective cohort study. Can. Urol. Assoc. J. 2014, 8, E600–E604. [Google Scholar] [CrossRef] [Green Version]
- Matulewicz, R.S.; Sharma, V.; McGuire, B.B.; Oberlin, D.T.; Perry, K.T.; Nadler, R.B. The effect of surgical duration of transurethral resection of bladder tumors on postoperative complications: An analysis of ACS NSQIP data. Urol. Oncol. 2015, 33, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, P.L.; Vargas, H.A.; Karlo, C.A.; Lakhman, Y.; Zheng, J.; Moskowitz, C.S.; Al-Ahmadie, H.A.; Sala, E.; Bochner, B.H.; Hricak, H. Intradiverticular bladder cancer: CT imaging features and their association with clinical outcomes. Clin. Imaging 2015, 39, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, J.R.; McCormick, B.; Wang, L.; Cohen, P.; Sun, D.; Penson, D.F.; Smith, J.A.; Clark, P.E.; Cookson, M.S.; Barocas, D.A.; et al. Short term complications from transurethral resection of bladder tumor. Can. J. Urol. 2016, 23, 8198–8203. [Google Scholar]
- Cornu, J.N.; Herrmann, T.; Traxer, O.; Matlaga, B. Prevention and Management Following Complications from Endourology Procedures. Eur. Urol. Focus 2016, 2, 49–59. [Google Scholar] [CrossRef]
- Bolat, D.; Gunlusoy, B.; Degirmenci, T.; Ceylan, Y.; Polat, S.; Aydin, E.; Aydogdu, O.; Kozacioglu, Z. Comparing the short-term outcomes and complications of monopolar and bipolar transurethral resection of non-muscle invasive bladder cancers: A prospective, randomized, controlled study. Arch. Esp. Urol. 2016, 69, 225–233. [Google Scholar] [CrossRef]
- Bansal, A.; Sankhwar, S.; Goel, A.; Kumar, M.; Purkait, B.; Aeron, R. Grading of complications of transurethral resection of bladder tumor using Clavien-Dindo classification system. Indian. J. Urol. 2016, 32, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Weber, R.; Patel, D.; Lowrance, W.; Mellis, A.; Cookson, M.; Lang, M.; Barocas, D.; Chang, S.; Newberger, E.; et al. A 10-Item Checklist Improves Reporting of Critical Procedural Elements during Transurethral Resection of Bladder Tumor. J. Urol. 2016, 196, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Konishi, T.; Washino, S.; Nakamura, Y.; Ohshima, M.; Saito, K.; Arai, Y.; Miyagawa, T. Risks and complications of transurethral resection of bladder tumors in patients receiving antiplatelet and/or anticoagulant therapy: A retrospective cohort study. BMC Urol. 2017, 17, 118. [Google Scholar] [CrossRef]
- Prader, R.; De Broca, B.; Chevallier, D.; Amiel, J.; Durand, M. Outcome of Transurethral Resection of Bladder Tumor: Does Antiplatelet Therapy Really Matter? Analysis of a Retrospective Series. J. Endourol. 2017, 31, 1284–1288. [Google Scholar] [CrossRef]
- Caras, R.J.; Lustik, M.B.; Kern, S.Q.; McMann, L.P.; Sterbis, J.R. Preoperative Albumin Is Predictive of Early Postoperative Morbidity and Mortality in Common Urologic Oncologic Surgeries. Clin. Genitourin. Cancer 2017, 15, e255–e262. [Google Scholar] [CrossRef]
- Naspro, R.; Lerner, L.B.; Rossini, R.; Manica, M.; Woo, H.H.; Calopedos, R.J.; Cracco, C.M.; Scoffone, C.M.; Herrmann, T.R.; de la Rosette, J.J.; et al. Perioperative antithrombotic therapy in patients undergoing endoscopic urologic surgery: Where do we stand with current literature? Minerva. Urol. Nefrol. 2018, 70, 126–136. [Google Scholar]
- Suskind, A.M.; Zhao, S.; Walter, L.C.; Boscardin, W.J.; Finlayson, E. Mortality and Functional Outcomes After Minor Urological Surgery in Nursing Home Residents: A National Study. J. Am. Geriatr. Soc. 2018, 66, 909–915. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Secco, S.; Macchi, V.; Porzionato, A.; De Caro, R.; Artibani, W. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur. Urol. 2009, 56, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Broer, T.; Bal, R.; Pickersgill, M. Problematisations of Complexity: On the Notion and Production of Diverse Complexities in Healthcare Interventions and Evaluations. Sci. Cult. (Lond.) 2017, 26, 135–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, C.; Bedford, T.; Cooke, R.M.; Hanea, A.M.; Morales-Napoles, O. Expert judgement for dependence in probabilistic modelling: A systematic literature review and future research directions. Eur. J. Operat. Res. 2017, 258, 801–819. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.I.; Brausi, M.; Clark, P.E.; Cookson, M.S.; Grossman, H.B.; Khochikar, M.; Kiemeney, L.A.; Malavaud, B.; Sanchez-Salas, R.; Soloway, M.S.; et al. Epidemiology, prevention, screening, diagnosis, and evaluation: Update of the ICUD-SIU joint consultation on bladder cancer. World J. Urol. 2019, 37, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Croskerry, P. From mindless to mindful practice—Cognitive bias and clinical decision making. N. Engl. J. Med. 2013, 368, 2445–2448. [Google Scholar] [CrossRef] [Green Version]
- Peard, L.; Goodwin, J.; Hensley, P.; Dugan, A.; Bylund, J.; Harris, A.M. Examining and Understanding Value: The Impact of Preoperative Characteristics, Intraoperative Variables, and Postoperative Complications on Cost of Robot-Assisted Laparoscopic Radical Prostatectomy. J. Endourol. 2019, 33, 541–548. [Google Scholar] [CrossRef]
- Bromwich, D. Plenty to worry about: Consent, control, and anxiety. Am. J. Bioeth. 2012, 12, 35–36. [Google Scholar] [CrossRef]
- Wiseman, O.J.; Wijewardena, M.; Calleary, J.; Masood, J.; Hill, J.T. ’Will you be doing my operation doctor?’ Patient attitudes to informed consent. Ann. R. Coll. Surg. Engl. 2004, 86, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Rutjes, A.W.; Reitsma, J.B.; Hooft, L.; Bossuyt, P.M. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013, 185, E537–E544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalgaard, L.P.; Zare, R.; Gaya, J.M.; Redorta, J.P.; Roumiguie, M.; Filleron, T.; Malavaud, B. Prospective evaluation of the performances of narrow-band imaging flexible videoscopy relative to white-light imaging flexible videoscopy, in patients scheduled for transurethral resection of a primary NMIBC. World J. Urol. 2019, 37, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, R.T. Multivariate statistical analysis for pathologist. Part I, The logistic model. Am. J. Clin. Pathol. 1996, 105, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [Green Version]







| Domain of Interest | Feature | Number of Items | Median Score, (95%CI) | Mann–Whitney U-Test | |
|---|---|---|---|---|---|
| TURBT Unlikely to Be Complex (n = 73) | TURBT Likely to Be Complex (n = 58) | ||||
| Patient’s characteristics | Age | 3 | 1 (1–1) | 1 (1–1) | n.s. (p = 0.85) |
| Sex | 2 | 1 (1–1) | 1 (1–2) | n.s. (p = 0.72) | |
| Patient’s history | 12 | 1 (1–2) | 2 (1–2) | n.s. (p = 0.07) | |
| Tumour’s characteristics | Number | 3 | 1 (1–1) | 3 (3–4) | p = 0.002 |
| Location | 10 | 3 (2–3) | 4 (3–4) | p < 0.0001 | |
| Size | 5 | 2 (1–3) | 3 (3–3) | p < 0.0001 | |
| Structure | 5 | 2 (2–3) | 2 (2–3) | n.s. (p = 0.97) | |
| Bladder Anatomy | 8 | 3 (2–3) | 3 (2–3) | n.s. (p = 0.82) | |
| Access to the Bladder cavity | 13 | 1 (1–3) | 3 (3–4) | p < 0.0001 | |
| Independent Variables | Regression Coefficient | Std. Error | z | p > |z| | 95% CI of the Regression Coefficient | |
|---|---|---|---|---|---|---|
| Patient History | 0.99 | 0.32 | 3.11 | 0.002 | 0.37 | 1.61 |
| Tumour Number | 0.96 | 0.23 | 4.18 | 0.000 | 0.51 | 1.41 |
| Main Tumour Location | 1.44 | 0.33 | 4.42 | 0.000 | 0.80 | 2.09 |
| Main Tumour Size | 1.04 | 0.26 | 3.98 | 0.000 | 0. 53 | 1.55 |
| Access | 1.10 | 0.26 | 4.31 | 0.000 | 0. 60 | 1.60 |
| Intercept value | −13.34 | 2.31 | −5.77 | 0.000 | −17.87 | −8.81 |
| Patient’s Characteristics | Tumour’s Characteristics | ||||
|---|---|---|---|---|---|
| Weight-Adjusted Scores | Medical History | Bladder Access | Number | Size | Location |
| 1 | No Relevant History | No relevant features | 1–3 | <3 cm | |
| 1.5 | Trigon | ||||
| 2 | Hip Surgery Radical Prostatectomy Repeated TURBT (>3) Prior Bladder perforation MMC or BCG instillations UTI | Large bladder (>500 mL) Irregular bladder wall, Trabeculations | Recent TURBT (second-look) | ||
| 3 | Obese BMI > 30 Pelvic Radiation Any open bladder surgery Bleeding disorder or Coumadin or Anti-aggregant | Urethral stricture High or narrow bladder neck Large Median lobe Large prostate (60–90 mL) Small bladder (100–250 mL) Female prolapse or cystocele | 4–10 | 3–5 cm Large micropapillary area or suspicious for CIS (>5 cm2) | Prostatic urethra Bladder neck Lateral wall |
| 4 | ASA class 4–5 | Not amenable to lithotomy position Very small bladder (<100 mL) Very large prostate (>90 mL) Bladder hernia Thin bladder wall | >10 | >5 cm | |
| 4.5 | Posterior or Anterior wall Ureteric orifice | ||||
| 6 | Dome Anticipate obturator jerk Diverticulum | ||||
| Expert | Country | Age | Urology * (Years) | Oncology * (Years) | FEBU | PhD | Head of Urology ** | National Association of Urology | European Association of Urology |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 36 | 4 | 2 | - | - | 0 | Member NMIBC guidelines panel | Member |
| 2 | F | 38 | 5 | 3 | Yes | Yes | - | Board member NMIBC guidelines panel | Chairman YAU Board member YOU & ESOU |
| 3 | CZ | 39 | 14 | - | Yes | Yes | - | Member | Member |
| 4 | D | 45 | 19 | 14 | Yes | Yes | 6 | Board Member in charge of Research | Vice-Chairman NMIBC guidelines panel |
| 5 | UK | 53 | 20 | 20 | Yes | - | 0 | Member | Member NMIBC guidelines panel |
| 6 | F | 58 | 26 | 26 | - | Yes | - | Member | Board Member ESOU |
| 7 | CZ | 58 | 27 | 22 | - | Yes | 10 | President of National Urological Society | Chairman NMIBC guidelines panel Member Education office of the ESU |
| 8 | F | 59 | 26 | 25 | Yes | Yes | 5 | Member | EAU Board Member ESU Member |
| 9 | E | 61 | 33 | 20 | Yes | Yes | 2 | Member | EAU Board member Director of ESU NMIBC Guidelines panel |
| 10 | NL | 62 | 28 | 28 | - | Yes | 22 | Chairman bladder cancer guidelines office | Chairman MIBC guidelines panel, ESU Member |
| Domains | Question | Likert Scores |
|---|---|---|
| Patient and tumour and bladder characteristics | How likely is this characteristic to negatively impact TURBT, that is, to result in incomplete resection or prolonged surgery (>1 h) or significant intra- or postoperative complications (Clavien-Dindo Grade III and higher)? | (1) It is very unlikely to impact TURBT |
| (2) It is unlikely to impact TURBT | ||
| (3) It may occasionally impact TURBT | ||
| (4) It is likely to impact TURBT | ||
| (5) It is very likely to impact TURBT | ||
| Surgical Environment | How likely is the following element of the surgical environment to influence the risk of TURBT resulting in either three situations, i.e., incomplete resection according to the operator, or prolonged surgery (>1 h) or significant intra- (bleeding that requires transfusion, laparotomy) or postoperative complications (Clavien-Dindo Grade III and higher)? | (1) It is very likely to reduce the risk |
| (2) It is likely to reduce the risk | ||
| (3) It is not expected to influence the risk in either way | ||
| (4) It is likely to increase the risk | ||
| (5) It is very likely to increase the risk | ||
| Clinical scenarios | In the following scenario, will TURBT result in incomplete resection or prolonged surgery (>1 h) or significant intra- or postoperative complications (Clavien-Dindo Grade III and higher)? | (1) This is very unlikely to happen |
| (2) This is unlikely to happen | ||
| (3) This may occasionally happen | ||
| (4) This is likely to happen | ||
| (5) This is very likely to happen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumiguié, M.; Xylinas, E.; Brisuda, A.; Burger, M.; Mostafid, H.; Colombel, M.; Babjuk, M.; Palou Redorta, J.; Witjes, F.; Malavaud, B. Consensus Definition and Prediction of Complexity in Transurethral Resection or Bladder Endoscopic Dissection of Bladder Tumours. Cancers 2020, 12, 3063. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12103063
Roumiguié M, Xylinas E, Brisuda A, Burger M, Mostafid H, Colombel M, Babjuk M, Palou Redorta J, Witjes F, Malavaud B. Consensus Definition and Prediction of Complexity in Transurethral Resection or Bladder Endoscopic Dissection of Bladder Tumours. Cancers. 2020; 12(10):3063. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12103063
Chicago/Turabian StyleRoumiguié, Mathieu, Evanguelos Xylinas, Antonin Brisuda, Maximillian Burger, Hugh Mostafid, Marc Colombel, Marek Babjuk, Joan Palou Redorta, Fred Witjes, and Bernard Malavaud. 2020. "Consensus Definition and Prediction of Complexity in Transurethral Resection or Bladder Endoscopic Dissection of Bladder Tumours" Cancers 12, no. 10: 3063. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12103063