FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Data Recording
2.3. Histopathology
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. World Health Organization Grades, Tumor Localization, and Extent of Resection
3.3. The MIB-1 Labeling Index in the Prediction of Intracranial Recurrent Meningioma
3.4. Association between the MIB-1 Labeling Index and Clinical, Tumor, and Laboratory Characteristics
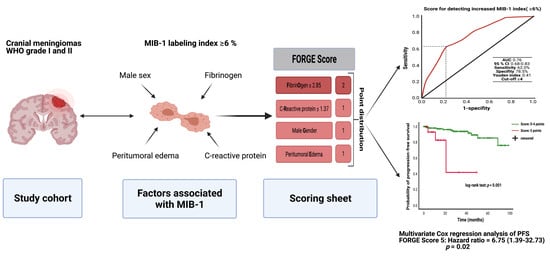
3.5. Predictive Score
3.6. The FORGE Score in the Prediction of Progression-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. 4), iv1–iv62. [Google Scholar] [CrossRef]
- Domingues, P.H.; Sousa, P.; Otero, Á.; Gonçalves, J.M.; Ruiz, L.; de Oliveira, C.; Lopes, M.C.; Orfao, A.; Tabernero, M.D. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro-Oncology 2014, 16, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Gogou, P.; Markoula, S.; Kyritsis, A.P. Management of meningiomas. Clin. Neurol. Neurosurg. 2010, 112, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pettersson-Segerlind, J.; Orrego, A.; Lönn, S.; Mathiesen, T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011, 76, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, P.J.; Brugal, G.; Baak, J.P. Proliferation markers in tumours: Interpretation and clinical value. J. Clin. Pathol. 1998, 51, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wach, J.; Brandecker, S.; Güresir, A.; Schuss, P.; Vatter, H.; Güresir, E. The impact of the MIB-1 index on facial nerve outcomes in vestibular schwannoma surgery. Acta Neurochir. 2020, 162, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Vranic, A.; Popovic, M.; Cör, A.; Prestor, B.; Pizem, J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: A study of 86 patients. Neurosurgery 2010, 67, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, S.; Kawai, K.; Nakatomi, H.; Saito, N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J. Neurosurg. 2012, 117, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Kim, K.H.; Lee, E.H.; Lee, Y.M.; Lee, S.H.; Kim, H.D.; Kim, Y.Z. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J. Neurosurg. 2014, 121, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Song, S.Y.; Jiang, J.B.; Wang, T.J.; Yan, C.X. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine 2020, 99, e18644. [Google Scholar] [CrossRef]
- Antinheimo, J.; Haapasalo, H.; Haltia, M.; Tatagiba, M.; Thomas, S.; Brandis, A.; Sainio, M.; Carpen, O.; Samii, M.; Jääskeläinen, J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J. Neurosurg. 1997, 87, 610–614. [Google Scholar] [CrossRef]
- Roser, F.; Nakamura, M.; Bellinzona, M.; Ritz, R.; Ostertag, H.; Tatagiba, M.S. Proliferation potential of spinal meningiomas. Eur. Spine J. 2006, 15, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.W.; Ulmer, S.; Harris, G.J. Brain tumor imaging in clinical trials. AJNR Am. J. Neuroradiol. 2008, 29, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.W.; Kim, M.S.; Kim, S.W.; Chang, C.H.; Kim, O.L. Peritumoral brain edema in meningiomas: Correlation of radiologic and pathologic features. J. Korean Neurosurg. Soc. 2011, 49, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Apallas, S.; Schneider, M.; Güresir, A.; Schuss, P.; Herrlinger, U.; Vatter, H.; Güresir, E. Baseline Serum C-Reactive Protein and Plasma Fibrinogen-Based Score in the Prediction of Survival in Glioblastoma. Front. Oncol. 2021, 11, 653614. [Google Scholar] [CrossRef]
- Majores, M.; Schick, V.; Engels, G.; Fassunke, J.; Elger, C.E.; Schramm, J.; Blumcke, I.; Becker, A.J. Mutational and immunohistochemical analysis of ezrin-, radixin-, moesin (ERM) molecules in epilepsy-associated glioneuronal lesions. Acta Neuropathol. 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Majores, M.; von Lehe, M.; Fassunke, J.; Schramm, J.; Becker, A.J.; Simon, M. Tumor recurrence and malignant progression of gangliogliomas. Cancer 2008, 113, 3355–3363. [Google Scholar] [CrossRef]
- Schneider, M.; Borger, V.; Güresir, A.; Becker, A.; Vatter, H.; Schuss, P.; Güresir, E. High Mib-1-score correlates with new cranial nerve deficits after surgery for frontal skull base meningioma. Neurosurg. Rev. 2021, 44, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lemée, J.M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert Rev. Neurother. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dundamadappa, S.K.; Thangasamy, S.; Flood, T.; Moser, R.; Smith, T.; Cauley, K.; Takhtani, D. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am. J. Roentgenol. 2014, 202, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Pavelin, S.; Becic, K.; Forempoher, G.; Mrklic, I.; Pogorelic, Z.; Titlic, M.; Andelinovic, S. Expression of Ki-67 and p53 in meningiomas. Neoplasma 2013, 60, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Zhang, C.; Zou, Y.; Lu, C.; Hu, H.; Qian, J.; Jiang, L.; Hu, G. Tissue thioredoxin-interacting protein expression predicted recurrence in patients with meningiomas. Int. J. Clin. Oncol. 2017, 22, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Coons, S.W.; Johnson, P.C. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J. Neuropathol. Exp. Neurol. 1993, 52, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Skaland, I.; Winther, T.L.; Salvesen, Ø.; Torp, S.H. Correlation Between Digital and Manual Determinations of Ki-67/MIB-1 Proliferative Indices in Human Meningiomas. Int. J. Surg. Pathol. 2020, 28, 273–279. [Google Scholar] [CrossRef]
- Kasuya, H.; Kubo, O.; Tanaka, M.; Amano, K.; Kato, K.; Hori, T. Clinical and radiological features related to the growth potential of meninigioma. Neurosurg. Rev. 2006, 29, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, A.; Fujimaki, T.; Sasaki, T.; Nagashima, T.; Ide, T.; Asai, A.; Matsuura, R.; Utsunomiya, H.; Kirino, T. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol. 1996, 91, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Jimbo, M.; Yamamoto, M.; Umebara, Y.; Hagiwara, S.; Kubo, O. MIB-1 staining index and peritumoral brain edema of meningiomas. Cancer 1996, 78, 133–143. [Google Scholar] [CrossRef]
- Berhouma, M.; Jacquesson, T.; Jouanneau, E.; Cotton, F. Pathogenesis of peri-tumoral edema in intracranial meningiomas. Neurosurg. Rev. 2019, 42, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Kshettry, V.R.; Selman, W.R.; Bambakidis, N.C. Peritumoral brain edema in intracranial meningiomas: The emergence of vascular endothelial growth factor-directed therapy. Neurosurg. Focus 2013, 35, E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, H.; Hama, S.; Taniguchi, E.; Sugiyama, K.; Arita, K.; Kurisu, K. Peritumoral brain edema associated with meningioma: Influence of vascular endothelial growth factor expression and vascular blood supply. Cancer 1999, 85, 936–944. [Google Scholar] [CrossRef]
- Nassehi, D. Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema. Dan Med. J. 2013, 60, B4626. [Google Scholar]
- Nayak, L.; Iwamoto, F.M.; Rudnick, J.D.; Norden, A.D.; Lee, E.Q.; Drappatz, J.; Omuro, A.; Kaley, T.J. Atypical and anaplastic meningiomas treated with bevacizumab. J. Neurooncol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Bitzer, M.; Wöckel, L.; Luft, A.R.; Wakhloo, A.K.; Petersen, D.; Opitz, H.; Sievert, T.; Ernemann, U.; Voigt, K. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J. Neurosurg. 1997, 87, 368–373. [Google Scholar] [CrossRef]
- Salpietro, F.M.; Alafaci, C.; Lucerna, S.; Iacopino, D.G.; Todaro, C.; Tomasello, F. Peritumoral edema in meningiomas: Microsurgical observations of different brain tumor interfaces related to computed tomography. Neurosurgery 1994, 35, 638–641. [Google Scholar] [CrossRef]
- Todo, T.; Adams, E.F.; Rafferty, B.; Fahlbusch, R.; Dingermann, T.; Werner, H. Secretion of interleukin-6 by human meningioma cells: Possible autocrine inhibitory regulation of neoplastic cell growth. J. Neurosurg. 1994, 81, 394–401. [Google Scholar] [CrossRef]
- Dubel, G.J.; Ahn, S.H.; Soares, G.M. Contemporary endovascular embolotherapy for meningioma. Semin. Intervent. Radiol. 2013, 30, 263–277. [Google Scholar] [PubMed] [Green Version]
- Huang, R.Y.; Bi, W.L.; Griffith, B.; Kaufmann, T.J.; Ia Fougere, C.; Schmidt, N.O.; Tonn, J.C.; Vogelbaum, M.A.; Wen, P.Y.; Aldape, K.; et al. Imaging and diagnostics advances for intracranial meningiomas. Neuro-Oncology 2019, 21 (Suppl. 1), i44–i61. [Google Scholar] [CrossRef] [PubMed]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar] [PubMed]
- Saija, A.; Princi, P.; Lanza, M.; Scalese, M.; Aramnejad, E.; De Sarro, A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995, 56, 775–784. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ahsworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.Y.F.; Leung, R.Y.H.; Ong, K.L.; Cheung, B.M.Y. Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene -572C>G polymorphism in subjects with and without hypertension. J. Hum. Hypertens. 2007, 21, 875–882. [Google Scholar] [CrossRef]
- Proctor, D.T.; Huang, J.; Lama, S.; Albakr, A.; Van Marle, G.; Sutherland, G.R. Tumor-associated macrophage infiltration in meningioma. Neurooncol. Adv. 2019, 1, vdz018. [Google Scholar] [CrossRef]
- Lisi, L.; Ciotti, G.M.; Braun, D.; Kalinin, S.; Curro, D.; Dello Russo, C.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.L.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, G.; Zhang, J.; Zhang, G.; Lin, Y.; Lin, Z.; Gu, J.; Kang, D.; Ding, C. A Novel Scoring System Based on Preoperative Routine Blood Test in Predicting Prognosis of Atypical Meningioma. Front. Oncol. 2020, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Gadient, R.A.; Otten, U.H. Interleukin-6 (IL-6)—A molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997, 52, 379–390. [Google Scholar] [CrossRef]
- Kishimoto, T. The biology of interleukin-6. Blood 1989, 74, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kishimoto, T. The biology and medical implications of interleukin-6. Cancer Immunol. Res. 2014, 2, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Boyle-Walsh, E.; Hashim, I.A.; Speirs, V.; Fraser, W.D.; White, M.C. Interleukin-6 (IL-6) production and cell growth of cultured human ameningiomas:-interactions with interleukin-1 beta (IL-1 beta) and interleukin-4 (IL-4) in vitro. Neurosci. Lett. 1994, 170, 129–132. [Google Scholar] [CrossRef]
- Jones, T.H.; Justice, S.K.; Timperley, W.R.; Royds, J.A. Effect of interleukin-1 and dexamethasone on interleukin-6 production and growth in human meningiomas. J. Pathol. 1997, 183, 460–468. [Google Scholar] [CrossRef]
- Mirian, C.; Skyrman, S.; Bartek, J., Jr.; Jensen, L.R.; Kihlström, L.; Förander, P.; Orrego, A.; Mathiesen, T. The Ki-67 Proliferation Index as a Marker of Time to Recurrence in Intracranial Meningioma. Neurosurgery 2020, 87, 1289–1298. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Kanungo, I.; Sudhir, S.; Chen, J.S.; Raleigh, D.R.; Magill, S.T.; McDermott, M.W.; Aghi, M.K. WHO grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front. Oncol. 2020, 10, 1522. [Google Scholar] [CrossRef]
- Ohba, S.; Kobayashi, M.; Horiguchi, T.; Onozuka, S.; Yoshida, K.; Ohira, T.; Kawase, T. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. J. Neurosurg. 2011, 114, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Soyuer, S.; Chang, E.L.; Selek, U.; Shi, W.; Maor, M.H.; DeMonte, F. Radiotherapy after surgery for benign cerebral meningioma. Radiother. Oncol. 2004, 71, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cha, Y.J.; Suh, S.H.; Lee, I.J.; Lee, K.S.; Hong, C.K.; Kim, J.W. Risk group-adapted adjuvant radiotherapy for WHO grade I and II skull base meningioma. J. Cancer Res. Clin. Oncol. 2019, 145, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Oya, S.; Ikawa, F.; Ichihara, N.; Wanibuchi, M.; Akiyama, Y.; Nakatomi, H.; Mikuni, N.; Narita, Y. Effect of adjuvant radiotherapy after subtotal resection for WHO grade I meningioma: A propensity score matching analysis of the Brain Tumor Registry of Japan. J. Neurooncol. 2021, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriya, J.; Tanino, M.A.; Takenami, T.; Endoh, T.; Urushido, M.; Kato, Y.; Wang, L.; Kimura, T.; Tsuda, M.; Nishihara, H.; et al. Rapid immunocytochemistry based on alternating current electric field using squash smear preparation of central nervous system tumors. Brain Tumor Pathol. 2016, 33, 13–18. [Google Scholar] [CrossRef]
- Terata, K.; Saito, H.; Nanjo, H.; Hiroshima, Y.; Ito, S.; Narita, K.; Akagami, Y.; Nakamura, R.; Konno, H.; Ito, A.; et al. Novel rapid-immunohistochemistry using an alternating current electric field for intraoperative diagnosis of sentinel lymph nodes in breast cancer. Sci. Rep. 2017, 7, 2810. [Google Scholar] [CrossRef] [Green Version]





| Median Age (IQR) (in y) | 61 (51–71) |
|---|---|
| Sex | |
| Female | 152 (73.1%) |
| Male | 56 (26.9%) |
| Median preoperative KPS (IQR) | 90 (80–100) |
| Tumor location | |
| Convexity | 65 (31.3%) |
| Falx | 36 (17.3%) |
| Sphenoid wing | 35 (16.8%) |
| Posterior fossa | 29 (13.9%) |
| Frontobasal | 27 (13.0%) |
| Others | 16 (7.7%) |
| Multiple meningioma | 18 (8.7%) |
| Peritumoral edema | 102 (49.0%) |
| Simpson grade | |
| Simpson grade I and II | 174 (83.7%) |
| Simpson grade ≥ III | 34 (16.3%) |
| WHO grade | |
| WHO grade I | 175 (84.1%) |
| WHO grade II | 33 (15.9%) |
| Variable | MIB-I ≥ 6% (n = 50) | MIB-I < 6% (n = 158) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 62.8 ± 16.0 | 60.3 ± 12.8 | 0.33 |
| Sex (female/male) | 35/23 | 117/33 | 0.02 |
| KPS (mean ± SD) | 86.8 ± 14.2 | 90.4 ± 11.1 | 0.10 |
| Diabetes (yes/no) | 9/41 | 16/142 | 0.21 |
| Smoking (yes/no; available in 204 patients) | 12/38 | 43/111 | 0.31 |
| BMI (mean ± SD) | 26.8 ± 6.1 | 27.6 ± 6.2 | 0.41 |
| ASA intake (yes/no) | 5/45 | 21/137 | 0.63 |
| Dexamethasone (yes/no) | 18/32 | 40/118 | 0.15 |
| Anticonvulsant drugs (yes/no) | 12/38 | 31/127 | 0.55 |
| Hemoglobin (mean ± SD) | 14.3 ± 1.5 | 14.0 ± 1.3 | 0.27 |
| MCV (mean ± SD) | 85.8 ± 8.6 | 87.6 ± 4.9 | 0.07 |
| Platelet count (mean ± SD) | 257.6 ± 78.5 | 278.2 ± 95.5 | 0.17 |
| MPV (mean ± SD) | 10.6 ± 0.9 | 12.8 ± 22.0 | 0.48 |
| Fibrinogen (mean ± SD) | 2.5 ± 0.9 | 3.2 ± 0.8 | <0.001 |
| C-reactive protein (mean ± SD) | 1.6 ± 2.1 | 4.3 ± 9.4 | 0.001 |
| Tumor size (mean ± SD, mm) | 39.8 ± 15.2 | 32.2 ± 15.1 | 0.002 |
| Peritumoral edema (yes/no) | 35/15 | 67/91 | 0.001 |
| Sinus invasion (yes/no) | 13/37 | 31/127 | 0.43 |
| Brain invasion (yes/no; available in 206 patients) | 6/44 | 6/150 | 0.08 |
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Sex (male/female) | 4.19 | 1.19–14.82 | 0.026 |
| KPS (<80/≥80) | 2.32 | 0.93–5.81 | 0.073 |
| Tumor size (<3.4 cm/≥3.4 cm) | 1.30 | 0.48–3.54 | 0.610 |
| Peritumoral edema (yes/no) | 2.90 | 0.99–8.47 | 0.049 |
| Fibrinogen (≤2.85 g/L/>2.85 g/L) | 3.52 | 1.14–10.90 | 0.029 |
| CRP (≤1.37 mg/I/>1.37 mg/I) | 4.90 | 1.23–19.50 | 0.024 |
| Diabetes (yes/no) | 1.29 | 0.44–3.80 | 0.649 |
| Brain invasion (yes/no) | 1.75 | 0.36–8.59 | 0.488 |
| Obesity (BMI ≥ 30.0/<30.0) | 1.93 | 0.55–6.81 | 0.305 |
| Preoperative corticosteroid medication (yes/no) | 1.26 | 0.43–3.65 | 0.676 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Age (<65/≥65) | 1.32 | 0.48–3.63 | 0.59 | 1.15 | 0.40–3.35 | 0.73 |
| KPS (≥80/<80) | 1.49 | 0.20–11.37 | 0.70 | 1.35 | 0.17–11.10 | 0.78 |
| WHO (I/II) | 1.48 | 0.47–4.67 | 0.50 | 1.77 | 0.43–7.33 | 0.43 |
| Score (<5/5) | 8.05 | 2.39–27.12 | 0.01 | 6.75 | 1.39–32.73 | 0.02 |
| Simpson Grade (≤II/>II) | 6.40 | 2.31–17.69 | <0.001 | 6.47 | 1.59–26.29 | 0.009 |
| Skull base meningioma (no/yes) | 1.68 | 0.61–4.65 | 1.26 | 0.34–4.67 | 0.73 | |
| Brain invasion (no/yes) | 3.90 | 0.86–17.71 | 0.08 | 2.47 | 0.42–14.56 | 0.32 |
| Dural sinus invasion (no/yes) | 2.65 | 0.87–8.05 | 0.09 | 1.32 | 0.30–5.93 | 0.72 |
| Multiple meningioma (no/yes) | 2.36 | 0.31–18.24 | 0.41 | 1.33 | 0.16–11.05 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Lampmann, T.; Güresir, Á.; Schuss, P.; Vatter, H.; Herrlinger, U.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers 2021, 13, 3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13143643
Wach J, Lampmann T, Güresir Á, Schuss P, Vatter H, Herrlinger U, Becker A, Hölzel M, Toma M, Güresir E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers. 2021; 13(14):3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13143643
Chicago/Turabian StyleWach, Johannes, Tim Lampmann, Ági Güresir, Patrick Schuss, Hartmut Vatter, Ulrich Herrlinger, Albert Becker, Michael Hölzel, Marieta Toma, and Erdem Güresir. 2021. "FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas" Cancers 13, no. 14: 3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13143643