Trends and Determinants in Uptake of Cervical Cancer Screening in Spain: An Analysis of National Surveys from 2017 and 2020
Abstract
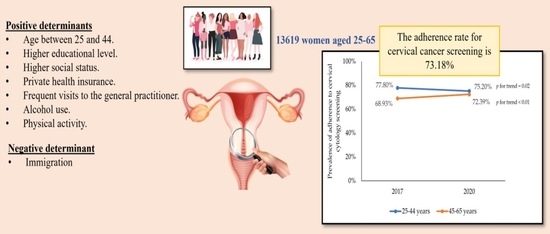
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Implications for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wentzensen, N.; Schiffman, M. Accelerating cervical cancer control and prevention. Lancet Public Health 2018, 3, e6–e7. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gao, K.; Gu, S.; You, L.; Qian, S.; Tang, M.; Wang, J.; Chen, K.; Jin, M. Worldwide Trends in Cervical Cancer Incidence and Mortality, with Predictions for the next 15 Years. Cancer 2021, 127, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- He, W.-Q.; Li, C. Recent Global Burden of Cervical Cancer Incidence and Mortality, Predictors, and Temporal Trends. Gynecol. Oncol. 2021, 163, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Cuenca, A.I.; Rodríguez-Borrego, M.-A.; Hidalgo-Lópezosa, P.; Rodríguez-Muñoz, P.M.; Martins, M.; Carmona-Torres, J.M. Prevalence and determinants in cytology testing for cervical cancer screening in Spain (2006-14). Eur. J. Public Health 2018, 28, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, W., Jr.; Peltecu, G.; Puiu, A.; Corha, A.; Cocîrṭă, E.; Cigăran, R.G.; Plante, M.; Jhingran, A.; Stang, K.; Gaffney, D.; et al. Cervical cancer in Eastern Europe: Review and proceedings from the cervical cancer research conference. Int. J. Gynecol. Cancer 2021, 31, 1061–1067. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Lavanya, G.; Grover, S.; Akinfenwa, C.A.; Carvalho, H.; Amornwichet, N. Incidence, treatment and outcomes of cervical cancer in low- and middle-income countries. Clin. Oncol. 2021, 33, e363–e371. [Google Scholar] [CrossRef]
- De Prez, V.; Jolidon, V.; Willems, B.; Cullati, S.; Burton-Jeangros, C.; Bracke, P. Cervical cancer screening programs and their context-dependent effect on inequalities in screening uptake: A dynamic interplay between public health policy and welfare state redistribution. Int. J. Equity Health 2021, 20, 211. [Google Scholar] [CrossRef]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical cancer screening: Past, present, and future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, May 2017. Wkly. Epidemiol. Rec. 2017, 92, 241–268. [Google Scholar]
- Gallagher, K.E.; LaMontagne, D.S.; Watson-Jones, D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018, 36, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. HPV-Vaccination and Cancer Cervical Screening in 53 WHO European Countries: An Update on Prevention Programs According to Income Level. Cancer Med. 2019, 8, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bouvard, V.; Wentzensen, N.; Mackie, A.; Berkhof, J.; Brotherton, J.; Giorgi-Rossi, P.; Kupets, R.; Smith, R.; Arrossi, S.; Bendahhou, K.; et al. The IARC Perspective on Cervical Cancer Screening. N. Engl. J. Med. 2021, 385, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.C.M.; et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health, Consumer Affairs and Social Welfare. Cribado Poblacional—Cancer Cervical; Ministry of Health, Consumer Affairs and Social Welfare: Madrid, Spain, 2022; Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/Cribado/CribadoCancerCervix.htm (accessed on 22 March 2022).
- Ministerio de Sanidad, Consumo y Bienestar Social. Orden SCB/480/2019, de 26 de Abril, por la Que se Modifican los Anexos I, III y VI del Real Decreto 1030/2006, de 15 de Septiembre, por el que se Establece la Cartera de Servicios Comunes del Sistema Nacional de Salud y el Procedimiento Para su Actualización; BOE: Madrid, Spain, 2019. [Google Scholar]
- Molina, A.; Moreno, J.; Peiró, R.; Arroyo, G.; Ibáñez, J.; Vanaclocha, M.; Binefa, G.; García, M.; Salas, D. Inequalities in access to cancer screening programmes in Spain and how to reduce them: Data from 2013 and 2020. Rev. Esp. Salud Publica 2021, 95, e1–e28. [Google Scholar]
- Castillo, M.; Astudillo, A.; Clavero, O.; Velasco, J.; Ibáñez, R.; de Sanjosé, S. Poor cervical cancer screening attendance and false negatives. A call for organized screening. PLoS ONE 2016, 11, e0161403. [Google Scholar] [CrossRef]
- Ministry of Health, Consumer Affairs and Social Welfare, National Institute of Statistics. Spanish National Health Survey 2017; Ministry of Health, Consumer Affairs and Social Welfare: Madrid, Spain, 2017; Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuesta2017.htm (accessed on 8 March 2022).
- Ministry of Health, Consumer Affairs and Social Welfare, National Institute of Statistics. European Health Interview Survey for Spain; Ministry of Health, Consumer Affairs and Social Welfare: Madrid, Spain, 2020; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/EncuestaEuropea/Enc_Eur_Salud_en_Esp_2020.htm (accessed on 8 March 2022).
- Ministry of Health, Consumer Affairs and Social Welfare, National Institute of Statistics. Spanish National Health Survey 2017: Methodology; Ministry of Health, Consumer Affairs and Social Welfare: Madrid, Spain, 2017; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE17_Metodologia.pdf (accessed on 19 March 2022).
- Ministry of Health, Consumer Affairs and Social Welfare, National Institute of Statistics. European Health Interview Survey for Spain 2020: Methodology; Ministry of Health, Consumer Affairs and Social Welfare: Madrid, Spain, 2020; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/EncuestaEuropea/EncuestaEuropea2020/Metodologia_EESE_2020.pdf (accessed on 19 March 2022).
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C. Proposals for social class classification based on the Spanish National Classification of Occupations 2011 using neo-Weberian and neo-Marxist approaches 2011. Gac. Sanit. 2013, 27, 263–272. [Google Scholar] [CrossRef] [Green Version]
- International Labour Office. Rural-Urban Labour Statistics; International Labour Office: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Body Mass Index (BMI). 2021. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 19 March 2022).
- Zamorano-Leon, J.J.; López-de-Andres, A.; Álvarez-González, A.; Astasio-Arbiza, P.; López-Farré, A.J.; de-Miguel-Diez, J.; Jiménez-García, R. Reduction from 2011 to 2017 in adherence to breast cancer screening and non-improvement in the uptake of cervical cancer screening among women living in Spain. Maturitas 2020, 135, 27–33. [Google Scholar] [CrossRef]
- Spanish Society of Medical Oncology. Cervical Cancer. Available online: https://seom.org/info-sobre-el-cancer/cervix (accessed on 28 March 2022).
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Williams, J.; Rakovac, I.; Victoria, J.; Tatarinova, T.; Corbex, M.; Barr, B.; Rose, T.; Sturua, L.; Obreja, G.; Andreasyan, D.; et al. Cervical cancer testing among women aged 30-49 years in the WHO European Region. Eur. J. Public Health 2021, 31, 884–889. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Cancer Screening in the European Union (2017). Report on the Implementation of the Council Recommendation on Cancer Screening; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Tavasoli, S.M.; Kane, E.; Chiarelli, A.M.; Kupets, R. Women’s behaviors toward mammogram and Pap Test: Opportunities to increase cervical cancer screening participation rates among older women. Women's Health Issues 2018, 28, 42–50. [Google Scholar] [CrossRef]
- Johnson, N.L.; Head, K.J.; Scott, S.F.; Zimet, G.D. Persistent disparities in cervical cancer screening uptake: Knowledge and sociodemographic determinants of Papanicolaou and Human Papillomavirus testing among women in the United States. Public Health Rep. 2020, 135, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Rendle, K.A.; Schiffman, M.; Cheung, L.C.; Kinney, W.K.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Castle, P.E. Adherence patterns to extended cervical screening intervals in women undergoing Human Papillomavirus (HPV) and cytology cotesting. Prev. Med. 2018, 109, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Petkeviciene, J.; Ivanauskiene, R.; Klumbiene, J. Sociodemographic and lifestyle determinants of non-attendance for cervical cancer screening in Lithuania, 2006–2014. Public Health 2018, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV Self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef] [PubMed]
- Agide, F.D.; Garmaroudi, G.; Sadeghi, R.; Shakibazadeh, E.; Yaseri, M.; Koricha, Z.B.; Tigabu, B.M. A systematic review of the effectiveness of health education interventions to increase cervical cancer screening uptake. Eur. J. Public Health 2018, 28, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Murfin, J.; Irvine, F.; Meechan-Rogers, R.; Swift, A. Education, income and occupation and their influence on the uptake of cervical cancer prevention strategies: A systematic review. J. Clin. Nurs. 2020, 29, 393–415. [Google Scholar] [CrossRef]
- Willems, B.; Bracke, P. The education gradient in cancer screening participation: A consistent phenomenon across Europe? Int. J. Public Health 2018, 63, 93–103. [Google Scholar] [CrossRef]
- Ayenew, A.A.; Zewdu, B.F.; Nigussie, A.A. Uptake of cervical cancer screening service and associated factors among age-eligible women in Ethiopia: Systematic review and meta-analysis. Infect. Agents Cancer 2020, 15, 67. [Google Scholar] [CrossRef]
- Nunes, M.F.; Leite, A.H.; Dias, S.F. Inequalities in adherence to cervical cancer screening in Portugal. Eur. J. Cancer Prev. 2021, 30, 171–177. [Google Scholar] [CrossRef]
- Willems, B.; Cullati, S.; Prez, V.D.; Jolidon, V.; Burton-Jeangros, C.; Bracke, P. Cancer screening participation and gender stratification in Europe. J. Health Soc. Behav. 2020, 61, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhang, L.; Wang, L.; Zhang, M.; Zhao, Z.; Fang, L.; Cong, S.; Zhou, M.; Wang, L. Significant variations in the cervical cancer screening rate in china by individual-level and geographical measures of socioeconomic status: A multilevel model analysis of a nationally representative survey dataset. Cancer Med. 2018, 7, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.M.; Brewer, N.T.; Melvin, C.; Yackle, A.; Smith, J.S.; Ko, L.K.; Crawford, A.; Glanz, K. Reducing overuse of cervical cancer screening: A systematic review. Prev. Med. 2018, 116, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Caprioglio, A.; Castagno, R.; Ronco, G.; Segnan, N.; Giordano, L. Inequalities in cervical cancer screening utilisation and results: A comparison between italian natives and immigrants from disadvantaged countries. Health Policy 2017, 121, 1072–1078. [Google Scholar] [CrossRef]
- Barrera-Castillo, M.; Fernández-Peña, R.; Del Valle-Gómez, M.D.O.; Fernández-Feito, A.; Lana, A. Social integration and gynecologic cancer screening of immigrant women in Spain. Gac. Sanit. 2020, 34, 468–473. [Google Scholar] [CrossRef]
- Bucchi, D.; Chiavarini, M.; Bianconi, F.; Galeotti, M.E.; Gili, A.; Stracci, F. Immigration, screening, and cervical cancer incidence: An application of age-period-cohort analysis. Eur. J. Cancer Prev. 2019, 28, 529–536. [Google Scholar] [CrossRef]
- Ferdous, M.; Lee, S.; Goopy, S.; Yang, H.; Rumana, N.; Abedin, T.; Turin, T.C. Barriers to cervical cancer screening faced by immigrant women in Canada: A systematic scoping review. BMC Women’s Health 2018, 18, 165. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; Meischke, H.; Ko, L.K. The impact of medical tourism on cervical cancer screening among immigrant women in the U.S. BMC Women’s Health 2021, 21, 414. [Google Scholar] [CrossRef]
- Alam, Z.; Shafiee Hanjani, L.; Dean, J.; Janda, M. Cervical cancer screening among immigrant women residing in Australia: A systematic review. Asia. Pac. J. Public Health 2021, 33, 816–827. [Google Scholar] [CrossRef]
- National Institute of Statistics. Flow of Immigration from Abroad by Year, Country of Origin and Nationality. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=24295#!tabs-grafico (accessed on 28 March 2022).
- Zhao, G.; Okoro, C.A.; Li, J.; Town, M. Health insurance status and clinical cancer screenings among US adults. Am. J. Prev. Med. 2018, 54, e11–e19. [Google Scholar] [CrossRef]
- Garrido-Cumbrera, M.; Borrell, C.; Palència, L.; Espelt, A.; Rodríguez-Sanz, M.; Pasarín, M.I.; Kunst, A. Social class inequalities in the utilization of health care and preventive services in Spain, a country with a National Health System. Int. J. Health Serv. 2010, 40, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.; Silles, M.; O’Neill, C. The role of private medical insurance in socio-economic inequalities in cancer screening uptake in Ireland: Private insurance and medical screening in the Republic of Ireland. Health Econ. 2012, 21, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Fuzzell, L.N.; Perkins, R.B.; Christy, S.M.; Lake, P.W.; Vadaparampil, S.T. Cervical cancer screening in the United States: Challenges and potential solutions for underscreened groups. Prev. Med. 2021, 144, 106400. [Google Scholar] [CrossRef]
- Freund, K.M.; Reisinger, S.A.; LeClair, A.M.; Yoon, G.H.; Al-Najar, S.M.; Young, G.S.; González, E.T.; Oliveri, J.M.; Paskett, E.D. Insurance stability and cancer screening behaviors. Health Equity 2019, 3, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suk, R.; Hong, Y.-R.; Rajan, S.S.; Xie, Z.; Zhu, Y.; Spencer, J.C. Assessment of US preventive services task force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw. Open 2022, 5, e2143582. [Google Scholar] [CrossRef] [PubMed]
- Gyulai, A.; Nagy, A.; Pataki, V.; Tonté, D.; Ádány, R.; Vokó, Z. General practitioners can increase participation in cervical cancer screening—A model program in Hungary. BMC Fam. Pract. 2018, 19, 67. [Google Scholar] [CrossRef] [Green Version]
- Harder, E.; Hertzum-Larsen, R.; Frederiksen, K.; Kjær, S.K.; Thomsen, L.T. Non-participation in cervical cancer screening according to health, lifestyle and sexual behavior: A Population-Based Study of nearly 15,000 Danish women aged 23–45 years. Prev. Med. 2020, 137, 106119. [Google Scholar] [CrossRef]
- Pelullo, C.P.; Cantore, F.; Lisciotto, A.; Di Giuseppe, G.; Pavia, M. Organized breast and cervical cancer screening: Attendance and determinants in southern Italy. Cancers 2021, 13, 1578. [Google Scholar] [CrossRef]
- Issa, T.; Babi, A.; Azizan, A.; Alibekova, R.; Khan, S.A.; Issanov, A.; Chan, C.K.; Aimagambetova, G. Factors associated with cervical cancer screening behaviour of women attending gynaecological clinics in Kazakhstan: A Cross-Sectional study. Women’s Health 2021, 17, 17455065211004136. [Google Scholar] [CrossRef]
- Venturelli, F.; Sampaolo, L.; Carrozzi, G.; Zappa, M.; Giorgi Rossi, P. Associations between cervical, breast and colorectal cancer screening uptake, chronic diseases and health-related behaviours: Data from the Italian PASSI Nationwide Surveillance. Prev. Med. 2019, 120, 60–70. [Google Scholar] [CrossRef]
- Ng’ang’a, A.; Nyangasi, M.; Nkonge, N.G.; Gathitu, E.; Kibachio, J.; Gichangi, P.; Wamai, R.G.; Kyobutungi, C. predictors of cervical cancer screening among Kenyan women: Results of a nested case-control study in a nationally representative survey. BMC Public Health 2018, 18, 1221. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.-A.; Benkortbi, K.; Kenfack, B.; Tincho Foguem, E.; Sormani, J.; Wisniak, A.; Lemoupa Makajio, S.; Manga, E.; Vassilakos, P.; Petignat, P. Recruitment strategies to promote uptake of cervical cancer screening in the West Region of Cameroon. BMC Public Health 2022, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.A.; Smith, M.A.; Killen, J.; Sy, S.; Simms, K.T.; Canfell, K.; Kim, J.J. projected time to elimination of cervical cancer in the USA: A Comparative Modelling Study. Lancet Public Health 2020, 5, e213–e222. [Google Scholar] [CrossRef]
- Hall, M.T.; Simms, K.T.; Lew, J.-B.; Smith, M.A.; Brotherton, J.M.; Saville, M.; Frazer, I.H.; Canfell, K. the projected timeframe until cervical cancer elimination in Australia: A modelling study. Lancet Public Health 2019, 4, e19–e27. [Google Scholar] [CrossRef] [Green Version]




| Variables | Cervical Cytology | |||
|---|---|---|---|---|
| Total n (%) | Yes n = 9967 (%) | No n = 3652 (%) | p-Value | |
| Age group | <0.001 | |||
| 45–65 years old | 7789 (57.19) | 5499 (70.60) | 2290 (29.40) | |
| 25–44 years old | 5830 (42.81) | 4468 (76.64) | 1362 (23.36) | |
| Educational level | <0.001 | |||
| Without studies | 57 (0.42) | 21 (36.84) | 36 (63.16) | |
| Primary | 1877 (13.78) | 1118 (59.56) | 759 (40.44) | |
| Secondary | 7762 (56.99) | 5653 (72.83) | 2109 (27.17) | |
| University | 3923 (28.81) | 3175 (80.93) | 748 (19.07) | |
| Marital status | <0.001 | |||
| Single | 3235 (23.75) | 2234 (69.06) | 1001 (30.94) | |
| Married | 8134 (59.73) | 6158 (75.71) | 1976 (24.29) | |
| Widowed | 588 (4.32) | 348 (59.18) | 240 (40.82) | |
| Separated or divorced | 1662 (12.20) | 1227 (73.83) | 435 (26.17) | |
| Social class | <0.01 | |||
| Lower | 6179 (45.37) | 4178 (67.62) | 2001 (32.38) | |
| Middle | 4473 (32.84) | 3390 (75.79) | 1083 (24.21) | |
| Upper | 2967 (21.79) | 2399 (80.86) | 568 (19.14) | |
| Place of residence | 0.79 | |||
| Urban | 4336 (31.84) | 3167 (73.04) | 1169 (26.96) | |
| Rural | 9283 (68.16) | 6800 (73.25) | 2483 (26.75) | |
| Nationality | <0.001 | |||
| Spanish | 12,158 (89.27) | 8959 (73.69) | 3199 (26.31) | |
| Foreigner | 1461 (10.73) | 1008 (68.99) | 453 (31.01) | |
| Mental illness | <0.01 | |||
| No | 11,486 (84.34) | 8455 (73.61) | 3031 (26.39) | |
| Yes | 2133 (15.66) | 1512 (70.89) | 621 (29.11) | |
| Self-assessed state of health | 0.14 | |||
| Very good | 2982 (21.90) | 2194 (73.57) | 788 (26.43) | |
| Good | 7095 (52.10) | 5235 (73.78) | 1860 (26.22) | |
| Average | 2648 (19.44) | 1910 (72.13) | 738 (27.87) | |
| Bad | 685 (5.03) | 481 (70.22) | 204 (29.78) | |
| Very bad | 209 (1.53) | 147 (70.33) | 62 (29.67) | |
| Insurance status | ||||
| Public | 12,981 (95.32) | 6422 (72.58) | 3559 (27.42) | <0.001 |
| Private | 638 (4.68) | 545 (85.42) | 93 (14.58) | |
| Visits to the family doctor in the preceding 4 weeks | ||||
| No | 10,058 (73.85) | 7310 (72.68) | 2748 (27.32) | 0.03 |
| Yes | 3561 (26.15) | 2657 (74.61) | 904 (25.39) | |
| Body Mass Index | <0.001 | |||
| Normal weight | 73.27 (53.80) | 5581 (76.17) | 1746 (23.83) | |
| Underweight | 417 (3.06) | 317 (76.02) | 100 (23.98) | |
| Overweight | 3915 (28.75) | 2760 (70.50) | 1155 (29.50) | |
| Obesity | 1960 (14.39) | 1309 (66.79) | 651 (33.21) | |
| Tobacco habit | 0.28 | |||
| No | 9985 (73.32) | 7332 (73.43) | 2653 (26.57) | |
| Yes | 3634 (26.68) | 2635 (72.51) | 999 (27.49) | |
| Alcohol use in the previous year | <0.001 | |||
| No | 4825 (35.43) | 3266 (67.69) | 1559 (32.31) | |
| Yes | 8794 (64.57) | 6701 (76.20) | 2093 (23.80) | |
| Free time physical exercise | <0.001 | |||
| No | 4912 (36.07) | 3411 (69.44) | 1501 (30.56) | |
| Yes | 8707 (63.93) | 6556 (75.30) | 2151 (24.70) | |
| Variables | Cervical Cytology (n = 9967) | ||
|---|---|---|---|
| 2017 n = 5286 (%) | 2020 n = 4681 (%) | p-Value | |
| Educational level | |||
| Without studies | 13 (38.24) | 8 (34.78) | 0.79 |
| Primary | 624 (57.88) | 494 (61.83) | 0.08 |
| Secondary | 3030 (73.05) | 2623 (72.58) | 0.64 |
| University | 1619 (81.19) | 1556 (80.66) | 0.67 |
| Marital status | |||
| Single | 1152 (69.95) | 1082 (68.14) | 0.27 |
| Married | 3330 (75.10) | 2828 (76.43) | 0.16 |
| Widowed | 184 (57.50) | 164 (61.19) | 0.28 |
| Separated or divorced | 620 (72.68) | 607 (75.03) | 0.38 |
| Social class | |||
| Lower | 2283 (67.11) | 1895 (68.24) | 0.38 |
| Middle | 1765 (75.82) | 1625 (75.76) | 0.97 |
| Upper | 1238 (81.23) | 1161 (80.46) | 0.34 |
| Place of residence | |||
| Urban | 1643 (71.43) | 1524 (74.85) | 0.10 |
| Rural | 3643 (73.54) | 3157 (72.93) | 0.51 |
| Nationality | |||
| Spanish | 4899 (73.62) | 4060 (73.74) | 0.91 |
| Foreigner | 387 (64.29) | 621 (72.29) | <0.01 |
| Mental illness | |||
| No | 4432 (73.76) | 4023 (73.45) | 0.71 |
| Yes | 854 (68.59) | 658 (74.10) | 0.18 |
| Self-assessed state of health | |||
| Very good | 1090 (74.25) | 1104 (72.92) | 0.41 |
| Good | 2740 (73.72) | 2495 (73.86) | 0.89 |
| Average | 1094 (71.04) | 896 (73.65) | 0.14 |
| Bad | 271 (68.81) | 210 (72.41) | 0.28 |
| Very bad | 91 (67.91) | 56 (74.67) | 0.38 |
| Insurance status | |||
| Public | 5002 (72.29) | 4420 (72.91) | 0.43 |
| Private | 284 (84.78) | 261 (86.14) | 0.63 |
| Visits to the family doctor in the preceding 4 weeks | |||
| No | 3721 (72.72) | 3589 (72.64) | 0.93 |
| Yes | 1565 (73.23) | 1092 (76.69) | 0.25 |
| Body Mass Index | |||
| Normal weight | 2974 (76.63) | 2607 (75.65) | 0.33 |
| Underweight | 160 (71.43) | 157 (81.35) | 0.12 |
| Overweight | 1433 (69.36) | 1327 (71.77) | 0.09 |
| Obesity | 719 (66.39) | 590 (67.27) | 0.68 |
| Tobacco habit | |||
| No | 3826 (73.38) | 3506 (73.49) | 0.90 |
| Yes | 1460 (71.57) | 1175 (73.71) | 0.15 |
| Alcohol use in the previous year | |||
| No | 1773 (67.75) | 1493 (67.62) | 0.92 |
| Yes | 3513 (7516) | 3188 (76.69) | 0.31 |
| Free time physical exercise | |||
| No | 1856 (69.95) | 1555 (70.05) | 0.41 |
| Yes | 3430 (75.19) | 3126 (75.42) | 0.80 |
| Variables | OR (CI 95%) | OR a (CI 95%) | p-Value |
|---|---|---|---|
| Age group | |||
| 45–65 years old | Reference | Reference | |
| 25–44 years old | 1.37 (1.26–1.48) | 1.28 (1.18–1.39) | <0.001 |
| Educational level | |||
| Without studies | Reference | Reference | |
| Primary | 2.53 (1.46–4.36) | 2.20 (1.27–3.82) | <0.001 |
| Secondary | 4.60 (2.68–7.89) | 3.41 (1.98–5.89) | <0.01 |
| University | 7.28 (4.22–12.54) | 4.28 (2.46–7.45) | <0.001 |
| Marital status | |||
| Single | Reference | ||
| Married | 1.40 (1.28–1.53) | ||
| Widowed | 0.65 (0.54–0.78) | ||
| Separated or divorced | 1.26 (1.11–1.44) | ||
| Social class | |||
| Lower | Reference | Reference | |
| Middle | 1.50 (1.38–1.64) | 1.28 (1.17–1.40) | <0.001 |
| Upper | 2.02 (1.82–2.25) | 1.39 (1.22–1.58) | <0.001 |
| Place of residence | |||
| Urban | Reference | ||
| Rural | 1.01 (0.93–1.10) | ||
| Nationality | |||
| Spanish | Reference | Reference | |
| Foreigner | 0.76 (0.71–0.89) | 0.88 (0.78–0.95) | 0.04 |
| Mental illness | |||
| No | Reference | ||
| Yes | 0.87 (0.79–0.97) | ||
| Self-assessed state of health | |||
| Very good | Reference | ||
| Good | 1.01 (0.92–1.11) | ||
| Average | 0.93 (0.83–1.05) | ||
| Bad | 0.85 (0.71–1.01) | ||
| Very bad | 0.85 (0.63–1.16) | ||
| Insurance status | |||
| Public | Reference | Reference | |
| Private | 2.21 (1.77–2.78) | 1.67 (1.33–2.10) | <0.001 |
| Visits to the family doctor in the preceding 4 weeks | |||
| No | Reference | Reference | |
| Yes | 1.11 (1.01–1.21) | 1.25 (1.14–1.37) | <0.001 |
| Body Mass Index | |||
| Normal weight | Reference | ||
| Underweight | 0.99 (0.79–1.25) | ||
| Overweight | 0.75 (0.69–0.82) | ||
| Obesity | 0.63 (0.57–0.70) | ||
| Tobacco habit | |||
| No | Reference | ||
| Yes | 0.96 (0.88–1.04) | ||
| Alcohol use in the previous year | |||
| No | Reference | Reference | |
| Yes | 1.53 (1.41–1.65) | 1.29 (1.19–1.40) | <0.001 |
| Free time physical exercise | |||
| No | Reference | Reference | |
| Yes | 1.34 (1.24–1.45) | 1.17 (1.08–1.27) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portero de la Cruz, S.; Cebrino, J. Trends and Determinants in Uptake of Cervical Cancer Screening in Spain: An Analysis of National Surveys from 2017 and 2020. Cancers 2022, 14, 2481. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14102481
Portero de la Cruz S, Cebrino J. Trends and Determinants in Uptake of Cervical Cancer Screening in Spain: An Analysis of National Surveys from 2017 and 2020. Cancers. 2022; 14(10):2481. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14102481
Chicago/Turabian StylePortero de la Cruz, Silvia, and Jesús Cebrino. 2022. "Trends and Determinants in Uptake of Cervical Cancer Screening in Spain: An Analysis of National Surveys from 2017 and 2020" Cancers 14, no. 10: 2481. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14102481