Outcomes of Patients with Small Intestine Adenocarcinoma in a Canadian Province: A Retrospective Multi-Center Population-Based Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Definitions
2.3. Analysis
2.4. Cox Proportional Regression Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival
3.3. Early-Stage Disease
3.4. Advanced-Stage Disease
3.5. Cox Proportional Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lepage, C.; Bouvier, A.M.; Manfredi, S.; Dancourt, V.; Faivre, J. Incidence and management of primary malignant small bowel cancers: A well-defined French population study. Am. J. Gastroenterol. 2006, 101, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef]
- Chaiyasate, K.; Jain, A.K.; Cheung, L.Y.; Jacobs, M.J.; Mittal, V.K. Prognostic factors in primary adenocarcinoma of the small intestine: 13-year single institution experience. World J. Surg. Oncol. 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, Y.W.; Rha, S.Y.; Shin, S.J.; Chang, H.; Shim, H.S.; Roh, J.K. Adenocarcinoma of the small bowel at a single Korean institute: Management and prognosticators. J. Cancer Res. Clin. Oncol. 2010, 136, 387–394. [Google Scholar] [CrossRef]
- Inoue, Y.; Hayashi, M.; Satou, N.; Miyamoto, Y.; Hirokawa, F.; Asakuma, M.; Shimizu, T.; Kayano, H.; Yamamoto, M.; Yamana, H.; et al. Prognostic clinicopathological factors after curative resection of small bowel adenocarcinoma. J. Gastrointest. Cancer 2012, 43, 272–278. [Google Scholar] [CrossRef]
- Abrahams, N.A.; Halverson, A.; Fazio, V.W.; Rybicki, L.A.; Goldblum, J.R. Adenocarcinoma of the small bowel: A study of 37 cases with emphasis on histologic prognostic factors. Dis. Colon. Rectum. 2002, 45, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Huffman, B.M.; Jin, Z.; Yadav, S.; Patel, S.; Nagorney, D.M.; Truty, M.J.; McWilliams, R.R.; Halfdanarson, T.R.; Mahipal, A. Novel Prognostic Factors in Resected Small Bowel Adenocarcinoma. Clin. Colorectal. Cancer 2019, 18, 218–225. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Li, Y.; Liu, W.; Zhao, L.; Güngör, C.; Tan, F.; Zhou, Y. Specific survival nomograms based on SEER database for small intestine adenocarcinoma. Ann. Palliat. Med. 2021, 10, 7440–7457. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, X.; Zhang, J.; Zhao, B.; Chen, C.; Liu, X.; Cao, H.; Li, T.; Geng, R.; Wang, W.; et al. Nomograms predict survival of patients with small bowel adenocarcinoma: A SEER-based study. Int. J. Clin. Oncol. 2021, 26, 387–398. [Google Scholar] [CrossRef]
- Gu, Y.; Deng, H.; Wang, D.; Li, Y. Metastasis Pattern and Survival Analysis in Primary Small Bowel Adenocarcinoma: A SEER-Based Study. Front. Surg. 2021, 8, 759162. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J. Recent advances in the management of adenocarcinoma of the small intestine. Gastrointest Cancer Res. 2009, 3, 90–96. [Google Scholar]
- Lech, G.; Korcz, W.; Kowalczyk, E.; Słotwiński, R.; Słodkowski, M. Primary small bowel adenocarcinoma: Current view on clinical features, risk and prognostic factors, treatment and outcome. Scand. J. Gastroenterol. 2017, 52, 1194–1202. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Colina, A.; Hwang, H.; Wang, H.; Katz, M.; Fournier, K.; Serpas, V.; Thomas, J.; Sun, R.; Wolff, R.A.; et al. Metastatic small bowel adenocarcinoma: Role of metastasectomy and systemic chemotherapy. ESMO Open 2021, 6, 100132. [Google Scholar] [CrossRef] [PubMed]
- Rompteaux, P.; Gagnière, J.; Gornet, J.M.; Coriat, R.; Baumgaertner, I.; Lecomte, T.; Afchain, P.; Zaanan, A.; Pocard, M.; Bachet, J.B.; et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur. J. Surg. Oncol. 2019, 45, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Sakae, H.; Kanzaki, H.; Nasu, J.; Akimoto, Y.; Matsueda, K.; Yoshioka, M.; Nakagawa, M.; Hori, S.; Inoue, M.; Inaba, T.; et al. The characteristics and outcomes of small bowel adenocarcinoma: A multicentre retrospective observational study. Br. J. Cancer 2017, 117, 1607–1613. [Google Scholar] [CrossRef]
- Howe, J.R.; Karnell, L.H.; Menck, H.R.; Scott-Conner, C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985–1995. Cancer 1999, 86, 2693–2706. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, R.; Cui, Y.; Zhou, Y.; Wu, X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci. Rep. 2018, 8, 9453. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19673. [Google Scholar] [CrossRef] [Green Version]
- Vano, Y.A.; Oudard, S.; By, M.A.; Têtu, P.; Thibault, C.; Aboudagga, H.; Scotté, F.; Elaidi, R. Optimal cut-off for neutrophil-to-lymphocyte ratio: Fact or Fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE 2018, 13, e0195042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Sautes-Fridman, C.; Fridman, W.H. The immune response in cancer: From immunology to pathology to immunotherapy. Virchows Arch. 2015, 467, 127–135. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Yu, I.S.; Al-Hashami, Z.; Chapani, P.; Speers, C.; Davies, J.M.; Lim, H.J.; Renouf, D.J.; Gill, S.; Stuart, H.C.; Loree, J.M. Impact of Tumor Location on Patient Outcomes in Small Bowel Cancers. Clin. Colorectal Cancer. 2021, 1, S1533-002800129-8. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; McWilliams, R.R.; Donohue, J.H.; Quevedo, J.F. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am. J. Surg. 2010, 199, 797–803. [Google Scholar] [CrossRef]
- Mizushima, T.; Tamagawa, H.; Mishima, H.; Ikeda, K.; Fujita, S.; Akamatsu, H.; Ikenaga, M.; Onishi, T.; Fukunaga, M.; Fukuzaki, T.; et al. The effects of chemotherapy on primary small bowel cancer: A retrospective multicenter observational study in Japan. Mol. Clin. Oncol. 2013, 1, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.J.; Liu, Y.W.; Zhang, L.; Qiu, F.; Yu, F.; Zhan, Z.Y.; Feng, M.; Yan, J.; Zhao, J.G.; Xiong, J.P. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Horimatsu, T.; Nakayama, N.; Moriwaki, T.; Hirashima, Y.; Fujita, M.; Asayama, M.; Moriyama, I.; Nakashima, K.; Baba, E.; Kitamura, H.; et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int. J. Clin. Oncol. 2017, 22, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.J.; Yeh, C.N.; Chao, T.C.; Jan, Y.Y.; Chen, M.F. Prognostic factors of primary small bowel adenocarcinoma: Univariate and multivariate analysis. World J. Surg. 2006, 30, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Mamtani, R.; Giantonio, B.J.; Dempsey, D.T.; Fraker, D.L.; Drebin, J.A.; Karakousis, G.C.; Roses, R.E. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: A propensity score-matched analysis. Cancer 2016, 122, 693–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overman, M.J.; Kopetz, S.; Lin, E.; Abbruzzese, J.L.; Wolff, R.A. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol. 2010, 49, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Young, J.I.; Mongoue-Tchokote, S.; Wieghard, N.; Mori, M.; Vaccaro, G.M.; Sheppard, B.C.; Tsikitis, V.L. Treatment and Survival of Small-bowel Adenocarcinoma in the United States: A Comparison With Colon Cancer. Dis. Colon Rectum 2016, 59, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Duerr, D.; Ellard, S.; Zhai, Y.; Taylor, M.; Rao, S. A Retrospective Review of Chemotherapy for Patients with Small Bowel Adenocarcinoma in British Columbia. J. Cancer 2016, 7, 2290–2295. [Google Scholar] [CrossRef] [Green Version]
- Singhal, N.; Singhal, D. Adjuvant chemotherapy for small intestine adenocarcinoma. Cochrane Database Syst Rev. 2007, 18, CD005202. [Google Scholar] [CrossRef]



| Variables | All Patients N = 112 (%) | Early Stage Disease N = 51 (%) | Advanced Disease N = 61 (%) | p Value |
|---|---|---|---|---|
| Median age in years | 73 (IQR: 62–81) | 71 (IQR: 62–79) | 75 (IQR: 62–82) | 0.15 |
| Men | 53 (47) | 27 (53) | 26 (43) | 0.34 |
| Rural resident | 66 (59) | 29 (57) | 37 (61) | 0.70 |
| Comorbid illness * | 74 of 99 (75) | 37 of 46 (80) | 37 of 53 (70) | 0.25 |
| Secondary cancer * | 35 of 103 (34) | 15 of 48 (31) | 20 of 55 (36) | 0.67 |
| WHO performance status < 1 | 48 (45) | 26 (55) | 22 (37) | 0.08 |
| Location | ||||
| Duodenum | 53 (47) | 20 (39) | 33 (54) | 0.13 |
| Jejunum | 17 (15) | 9(18) | 8 (13) | 0.60 |
| Ileum | 26 (23) | 15 (29) | 11 (18) | 0.18 |
| Not specified | 16 (14) | 7 (14) | 9 (15) | 1.0 |
| Resection of primary tumor ± metastases | 63 (56) | 41 (80) | 22 (36) | 0.0001 |
| Resection of metastases | 9 (14) | 6 (15) | 3 (14) | 1.0 |
| T4 tumor | 50 (45) | 20 (39) | 30 (49) | 0.0005 |
| Node negative disease | 53 (47) | 32 (63) | 21 (34) | 0.015 |
| Stage | ||||
| I | 10 (9) | 10 (20) | - | |
| II | 25 (22) | 25 (49) | - | |
| III | 16 (14) | 16 (31) | - | |
| IV | 55 (49) | - | 55 (90) | |
| Not known ** | 6 (5) | 0 | 6 (10) | |
| Mean creatinine | 91.4 ± 75.3 | 97.3 ± 91.6 | 86.1 ± 58 | 0.50 |
| Mean albumin | 31.5 ± 7.7 | 33.1 ± 6.2 | 30.1 ± 8.6 | 0.11 |
| Mean alkaline phosphatase | 164 ± 153.4 | 102 ± 92.5 | 214 ± 173 | 0.001 |
| Mean WBC | 8.9 ± 3.6 | 8.0 ± 3.3 | 9.7 ± 3.6 | 0.02 |
| Mean hemoglobin | 113 ± 22 | 120 ± 22 | 108 ± 22 | 0.016 |
| Mean platelets | 310 ± 132 | 299 ± 111 | 320 ± 150 | 0.48 |
| Mean lymphocytes | 1.38 ± 0.71 | 1.39 ± 0.60 | 1.38 ± 0.81 | 0.95 |
| Mean neutrophils | 6.40 ± 3.32 | 5.54 ± 3.22 | 7.14 ± 3.18 | 0.03 |
| Mean neutrophil: lymphocyte | 6.1 ± 6.1 | 5.8 ± 8.0 | 6.3 ± 3.8 | 0.71 |
| Chemotherapy | ||||
| (neo)Adjuvant | 20 (18) | 18 (35) | 2 (3) | <0.0001 |
| Recurrent/metastatic disease | 39 (35) | 14 (28) | 25 (41) | 0.17 |
| Received radiation | 14 (13) | 6 (12) | 8 (13) | 1.0 |
| Palliative | 8 (57) | 2 (33) | 6 (75) | 0.28 |
| Variables | HR 95% CI | p |
|---|---|---|
| Age ≥ 70 years | 1.66 (1.07–2.57) | 0.02 |
| Male sex | 1.35 (0.90–2.04) | 0.15 |
| Comorbid illness | 0.92 (0.57–1.51) | 0.75 |
| Secondary cancer | 1.10 (0.70–1.72) | 0.68 |
| Rural residence | 1.04 (0.69–1.58) | 0.84 |
| H/O colorectal cancer | 0.90 (0.53–1.55) | 0.90 |
| WHO performance status > 1 | 2.50 (1.62–3.81) | <0.001 |
| Stage 4 disease | 4.32 (2.70–7.0) | <0.001 |
| Albumin < 35 g/L | 2.0 (1.20–3.38) | 0.01 |
| Creatinine > 120 | 1.70 (1.11–2.62) | 0.015 |
| Alkaline phosphatase > 140 | 2.38 (1.56–3.61) | <0.001 |
| Hemoglobin < 120 g/L | 1.22 (0.79–1.90) | 0.36 |
| Platelets > 450 | 1.65 (0.82–3.30) | 0.18 |
| Duodenum | 1.73 (1.15–2.62) | 0.008 |
| Neutrophil:lymphocyte ratio > 4.5 | 2.26 (1.44–3.55) | <0.001 |
| No chemotherapy | 1.45 (0.95–2.20) | 0.080 |
| No primary tumor resection ± metastasectomy | 4.0 (2.51–6.38) | <0.001 |
| Radiation therapy | 1.08 (0.61–1.91) | 0.78 |
| Variables | HR (95% CI) | p | HR (95%CI) | p Value |
|---|---|---|---|---|
| Age ≥ 70 years | 1.31 (0.78–2.22) | 0.31 | ||
| Age < 70 years | 1 | |||
| Men Women | 1.26 (0.77–2.10) 1 | 0.35 | ||
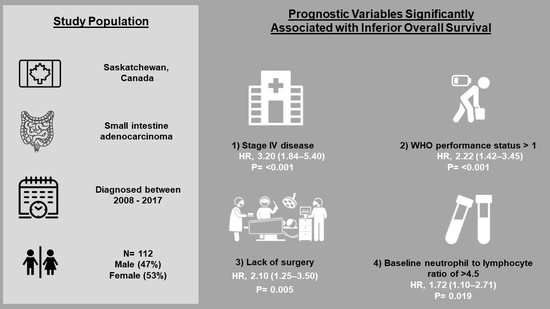
| WHO performance status > 1 | 2.01 (1.17–3.46) | 0.012 | 2.22 (1.42–3.45) | <0.001 |
| WHO performance status ≤ 1 | 1 | |||
| Stage 4 disease | 3.0 (1.74–5.16) | <0.001 | 3.20 (1.84–5.40) | <0.001 |
| Stage 1, 2, or 3 disease | 1 | |||
| Albumin < 35 g/L | 1.05 (0.56–1.97) | 0.87 | ||
| Albumin ≥ 35 g/L | 1 | |||
| Creatinine > 120 | 1.42 (0.76–2.66) | 0.27 | ||
| Creatinine ≤ 120 | 1 | |||
| Alkaline phosphatase > 140 | 1.47 (0.78–2.77) | 0.23 | ||
| Alkaline phosphatase ≤ 140 | 1 | |||
| Duodenum | 1.20 (0.68–2.10) | 0.53 | ||
| Jejunum, ileum, or not known | 1 | |||
| Neutrophil:lymphocyte ratio > 4.5 | 1.90 (1.10–3.28) | 0.02 | 1.72 (1.10–2.71) | 0.019 |
| Neutrophil:lymphocyte ratio ≤ 4.5 | 1 | |||
| No chemotherapy | 1.02 (0.56–1.84) | 0.96 | ||
| Received chemotherapy | 1 | |||
| No surgery | 1.96 (1.02–3.80) | 0.04 | 2.10 (1.25–3.50) | 0.005 |
| Surgery | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanko, E.; Le, D.; Mahmood, S.; Ginther, D.N.; Chalchal, H.I.; Kanthan, R.; Haider, K.; Zaidi, A.; Dueck, D.-A.; Ahmed, O.; et al. Outcomes of Patients with Small Intestine Adenocarcinoma in a Canadian Province: A Retrospective Multi-Center Population-Based Cohort Study. Cancers 2022, 14, 2581. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112581
Yanko E, Le D, Mahmood S, Ginther DN, Chalchal HI, Kanthan R, Haider K, Zaidi A, Dueck D-A, Ahmed O, et al. Outcomes of Patients with Small Intestine Adenocarcinoma in a Canadian Province: A Retrospective Multi-Center Population-Based Cohort Study. Cancers. 2022; 14(11):2581. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112581
Chicago/Turabian StyleYanko, Emma, Duc Le, Shazia Mahmood, David Nathan Ginther, Haji Ibraheem Chalchal, Rani Kanthan, Kamal Haider, Adnan Zaidi, Dorie-Anna Dueck, Osama Ahmed, and et al. 2022. "Outcomes of Patients with Small Intestine Adenocarcinoma in a Canadian Province: A Retrospective Multi-Center Population-Based Cohort Study" Cancers 14, no. 11: 2581. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112581