Online Learning of Finite and Infinite Gamma Mixture Models for COVID-19 Detection in Medical Images
Abstract
:1. Introduction
2. Related Works
3. Proposed Method
3.1. Model Specification: Finite Gamma Mixture Models
3.2. Model Learning
3.2.1. Deterministic Learning
- Initialization-step: Initialize the parameters of the Gamma model with initial values.
- 2.
- E-step: Compute the posterior probability as:
- 3.
- M-step: Update the model’s parameters under this condition:
3.2.2. Bayesian Learning
- Initialization
- Step t: For t=1,…
- (a)
- Generate
- (b)
- Generate from
3.2.3. Variational Learning
3.3. Infinite Gamma Mixture Model
3.4. Online Learning Algorithm
4. Experimental Results
4.1. Feature Extraction Step
4.2. Data Sets
4.3. Results Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, W.; Sallay, H.; Bouguila, N. Online Learning of Hierarchical Pitman-Yor Process Mixture of Generalized Dirichlet Distributions With Feature Selection. IEEE Trans. Neural Netw. Learn. Syst. 2017, 28, 2048–2061. [Google Scholar] [CrossRef]
- Bourouis, S.; Zaguia, A.; Bouguila, N.; Alroobaea, R. Deriving Probabilistic SVM Kernels From Flexible Statistical Mixture Models and its Application to Retinal Images Classification. IEEE Access 2019, 7, 1107–1117. [Google Scholar] [CrossRef]
- Fan, W.; Bouguila, N. Modeling and Clustering Positive Vectors via Nonparametric Mixture Models of Liouville Distributions. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 3193–3203. [Google Scholar] [CrossRef]
- Albuquerque, V.H.C.D.; Gupta, D.; Falco, I.D.; Sannino, G.; Bouguila, N. Special issue on Bio-inspired optimization techniques for Biomedical Data Analysis: Methods and applications. Appl. Soft Comput. 2020, 95, 106672. [Google Scholar] [CrossRef]
- Alroobaea, R.; Rubaiee, S.; Bourouis, S.; Bouguila, N.; Alsufyani, A. Bayesian inference framework for bounded generalized Gaussian-based mixture model and its application to biomedical images classification. Int. J. Imaging Syst. Technol. 2020, 30, 18–30. [Google Scholar] [CrossRef]
- Bourouis, S.; Zaguia, A.; Bouguila, N. Hybrid Statistical Framework for Diabetic Retinopathy Detection. In Proceedings of the Image Analysis and Recognition—15th International Conference, ICIAR 2018, Póvoa de Varzim, Portugal, 27–29 June 2018; pp. 687–694. [Google Scholar]
- Beckmann, C.; Woolrich, M.; Smith, S. Gaussian/Gamma mixture modeling of ICA/GLM spatial maps. Neuroimage 2003, 19. [Google Scholar] [CrossRef]
- Fan, W.; Bouguila, N.; Bourouis, S.; Laalaoui, Y. Entropy-based variational Bayes learning framework for data clustering. IET Image Process. 2018, 12, 1762–1772. [Google Scholar] [CrossRef]
- Najar, F.; Bourouis, S.; Zaguia, A.; Bouguila, N.; Belghith, S. Unsupervised Human Action Categorization Using a Riemannian Averaged Fixed-Point Learning of Multivariate GGMM. In Proceedings of the Image Analysis and Recognition—15th International Conference, ICIAR 2018, Póvoa de Varzim, Portugal, 27–29 June 2018; pp. 408–415. [Google Scholar]
- Sannino, G.; Bouguila, N.; Pietro, G.D.; Celesti, A. Artificial Intelligence for Mobile Health Data Analysis and Processing. Mob. Inf. Syst. 2019, 2019, 2673463:1–2673463:2. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, A.; Chung, M.; Bernheim, A.; Eber, C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin. Imaging 2020, 64, 35–42. [Google Scholar] [CrossRef]
- Parveen, N.; Sathik, M.M. Detection of pneumonia in chest X-ray images. J. Ray Sci. Technol. 2011, 19, 423–428. [Google Scholar] [CrossRef]
- Van Ginneken, B.; Stegmann, M.B.; Loog, M. Segmentation of anatomical structures in chest radiographs using supervised methods: A comparative study on a public database. Med. Image Anal. 2006, 10, 19–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, H.; van Ginneken, B.; Summers, R.M. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Trans. Med. Imaging 2016, 35, 1153–1159. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordienko, Y.; Gang, P.; Hui, J.; Zeng, W.; Kochura, Y.; Alienin, O.; Rokovyi, O.; Stirenko, S. Deep learning with lung segmentation and bone shadow exclusion techniques for chest X-ray analysis of lung cancer. In Proceedings of the International Conference on Computer Science, Engineering and Education Applications, San Francisco, CA, USA, 23–25 October 2018; pp. 638–647. [Google Scholar]
- Novikov, A.A.; Lenis, D.; Major, E.A. Fully convolutional architectures for multiclass segmentation in chest radiographs. IEEE Trans. Med. Imaging 2018, 37, 1865–1876. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Feng, J.; Wu, X.; Yan, S. A survey on deep learning-based fine-grained object classification and semantic segmentation. Int. J. Autom. Comput. 2017, 14, 119–135. [Google Scholar] [CrossRef]
- Xu, T.; Mandal, M.K.; Long, R.; Cheng, I.; Basu, A. An edge-region force guided active shape approach for automatic lung field detection in chest radiographs. Comput. Med. Imaging Graph. 2012, 36, 452–463. [Google Scholar] [CrossRef]
- Candemir, S.; Jaeger, S.; Palaniappan, K.; Musco, J.P.; Singh, R.K.; Xue, Z.; Karargyris, A.; Antani, S.; Thoma, G.; McDonald, C.J. Lung Segmentation in Chest Radiographs Using Anatomical Atlases With Nonrigid Registration. IEEE Trans. Med. Imaging 2014, 33, 577–590. [Google Scholar] [CrossRef]
- Da Nóbrega, R.V.M.; Filho, P.P.R.; Rodrigues, M.B.; da Silva, S.P.P.; Júnior, C.M.J.M.D.; de Albuquerque, V.H.C. Lung nodule malignancy classification in chest computed tomography images using transfer learning and convolutional neural networks. Neural Comput. Appl. 2020, 32, 11065–11082. [Google Scholar] [CrossRef]
- Zotin, A.; Hamad, Y.; Simonov, K.; Kurako, M. Lung boundary detection for chest X-ray images classification based on GLCM and probabilistic neural networks. In Proceedings of the 23rd International Conference KES Knowledge-Based and Intelligent Information & Engineering Systems, Budapest, Hungary, 4–6 September 2019; pp. 1439–1448. [Google Scholar]
- Melendez, J.; van Ginneken, B.; Maduskar, P.; Philipsen, R.H.H.M.; Reither, K.; Breuninger, M.; Adetifa, I.M.O.; Maane, R.; Ayles, H.; Sánchez, C.I. A Novel Multiple-Instance Learning-Based Approach to Computer-Aided Detection of Tuberculosis on Chest X-Rays. IEEE Trans. Med. Imaging 2015, 34, 179–192. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Li, P.; Jiang, L. Application of high-resolution CT images information in complicated infection of lung tumors. J. Infect. Public Health 2019, accepted. [Google Scholar] [CrossRef]
- Lassen, B.; Jacobs, C.; Kuhnigk, J.; Van Ginneken, B.; Van Rikxoort, E. Robust semi-automatic segmentation of pulmonary subsolid nodules in chest computed tomography scans. Phys. Med. Biol. 2015, 60, 1307. [Google Scholar] [CrossRef] [PubMed]
- Bouguila, N.; Almakadmeh, K.; Boutemedjet, S. A finite mixture model for simultaneous high-dimensional clustering, localized feature selection and outlier rejection. Expert Syst. Appl. 2012, 39, 6641–6656. [Google Scholar] [CrossRef]
- Llera, A.; Vidaurre, D.; Pruim, R.H.R.; Beckmann, C.F. Variational Mixture Models with Gamma or Inverse-Gamma Components. arXiv 2016, arXiv:1607.07573. [Google Scholar]
- Najar, F.; Bourouis, S.; Bouguila, N.; Belghith, S. Unsupervised learning of finite full covariance multivariate generalized Gaussian mixture models for human activity recognition. Multimed. Tools Appl. 2019, 78, 18669–18691. [Google Scholar] [CrossRef]
- Husmeier, D. The Bayesian Evidence Scheme for Regularizing Probability-Density Estimating Neural Networks. Neural Comput. 2000, 12, 2685–2717. [Google Scholar] [CrossRef]
- Husmeier, D.; Penny, W.D.; Roberts, S.J. An empirical evaluation of Bayesian sampling with hybrid Monte Carlo for training neural network classifiers. Neural Netw. 1999, 12, 677–705. [Google Scholar] [CrossRef]
- Bourouis, S.; Al-Osaimi, F.R.; Bouguila, N.; Sallay, H.; Aldosari, F.; Al Mashrgy, M. Bayesian inference by reversible jump MCMC for clustering based on finite generalized inverted Dirichlet mixtures. Soft Comput. 2018, 23, 5799–5813. [Google Scholar] [CrossRef]
- Congdon, P. Applied Bayesian Modelling; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Tan, S.L.; Nott, D.J. Variational approximation for mixtures of linear mixed models. J. Comput. Graph. Stat. 2014, 23, 564–585. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Sallay, H.; Bouguila, N.; Bourouis, S. Variational learning of hierarchical infinite generalized Dirichlet mixture models and applications. Soft Comput. 2016, 20, 979–990. [Google Scholar] [CrossRef]
- Elguebaly, T.; Bouguila, N. Generalized Gaussian mixture models as a nonparametric Bayesian approach for clustering using class-specific visual features. J. Vis. Commun. Image Represent. 2012, 23, 1199–1212. [Google Scholar] [CrossRef]
- Bourouis, S.; Laalaoui, Y.; Bouguila, N. Bayesian frameworks for traffic scenes monitoring via view-based 3D cars models recognition. Multimed. Tools Appl. 2019, 78, 18813–18833. [Google Scholar] [CrossRef]
- Bouguila, N.; Ziou, D. A Dirichlet process mixture of generalized Dirichlet distributions for proportional data modeling. IEEE Trans. Neural Netw. 2010, 21, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.F. On Recursive Estimation in Incomplete Data Models. Statistics 2000, 34, 27–51. [Google Scholar] [CrossRef]
- Sato, M. Online Model Selection Based on the Variational Bayes. Neural Comput. 2001, 13, 1649–1681. [Google Scholar] [CrossRef]
- Fan, W.; Bouguila, N. Online variational finite Dirichlet mixture model and its applications. In Proceedings of the 11th International Conference on Information Science, Signal Processing and their Applications—ISSPA 2012, Montreal, QC, Canada, 2–5 July 2012; pp. 448–453. [Google Scholar]
- Xie, J.; Jiang, Y.; Tsui, H. Segmentation of kidney from ultrasound images based on texture and shape priors. IEEE Trans. Med. Imaging 2005, 24, 45–57. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.S.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Pourghassem, H.; Ghassemian, H. Content-based medical image classification using a new hierarchical merging scheme. Comput. Med. Imaging Graph. 2008, 32, 651–661. [Google Scholar] [CrossRef]
- Cohen, J.P.; Morrison, P.; Dao, L. COVID-19 image data collection. arXiv 2020, arXiv:2003.11597. [Google Scholar]
- Zhao, J.; Zhang, Y.; He, X.; Xie, P. COVID-CT Dataset: A CT scan data set about COVID-19. arXiv 2020, arXiv:2003.13865. [Google Scholar]
- Sabhadiya, P.; Desai, V.; Sorathiya, N. COVID-CAM: A Method of Detection COVID using Active Map Classification, CNN and Deep Learning. Int. J. Comput. Appl. 2020, 176, 7–13. [Google Scholar] [CrossRef]
- Bouguila, N.; Ziou, D. Online clustering via finite mixtures of Dirichlet and minimum message length. Eng. Appl. Artif. Intell. 2006, 19, 371–379. [Google Scholar] [CrossRef]
- Fan, W.; Bouguila, N. Online Learning of a Dirichlet Process Mixture of Beta-Liouville Distributions Via Variational Inference. IEEE Trans. Neural Netw. Learn. Syst. 2013, 24, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Bourouis, S.; Channoufi, I.; Alroobaea, R.; Rubaiee, S.; Andejany, M.; Bouguila, N. Color object segmentation and tracking using flexible statistical model and level-set. Multimed. Tools Appl. 2020. [Google Scholar] [CrossRef]
- Smith, G.; Burns, I. Measuring texture classification algorithms. Pattern Recognit. Lett. 1997, 18, 1495–1501. [Google Scholar] [CrossRef]
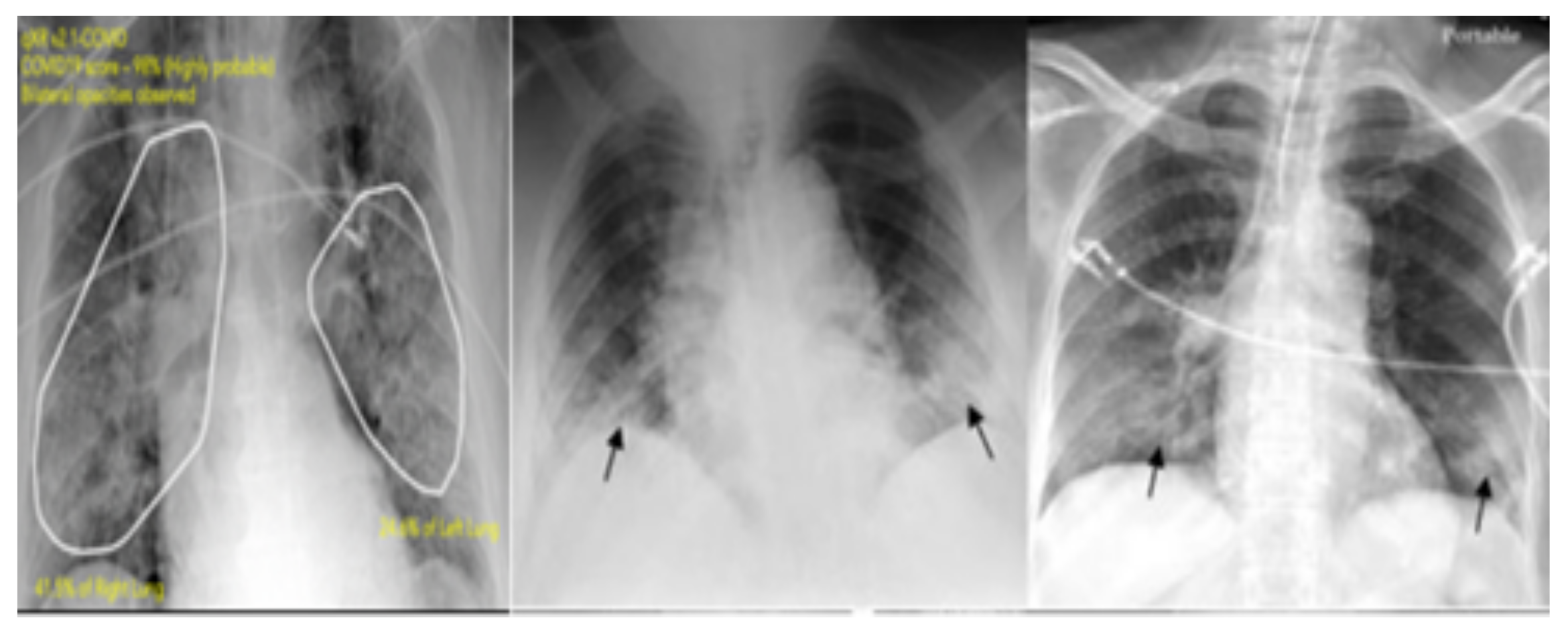
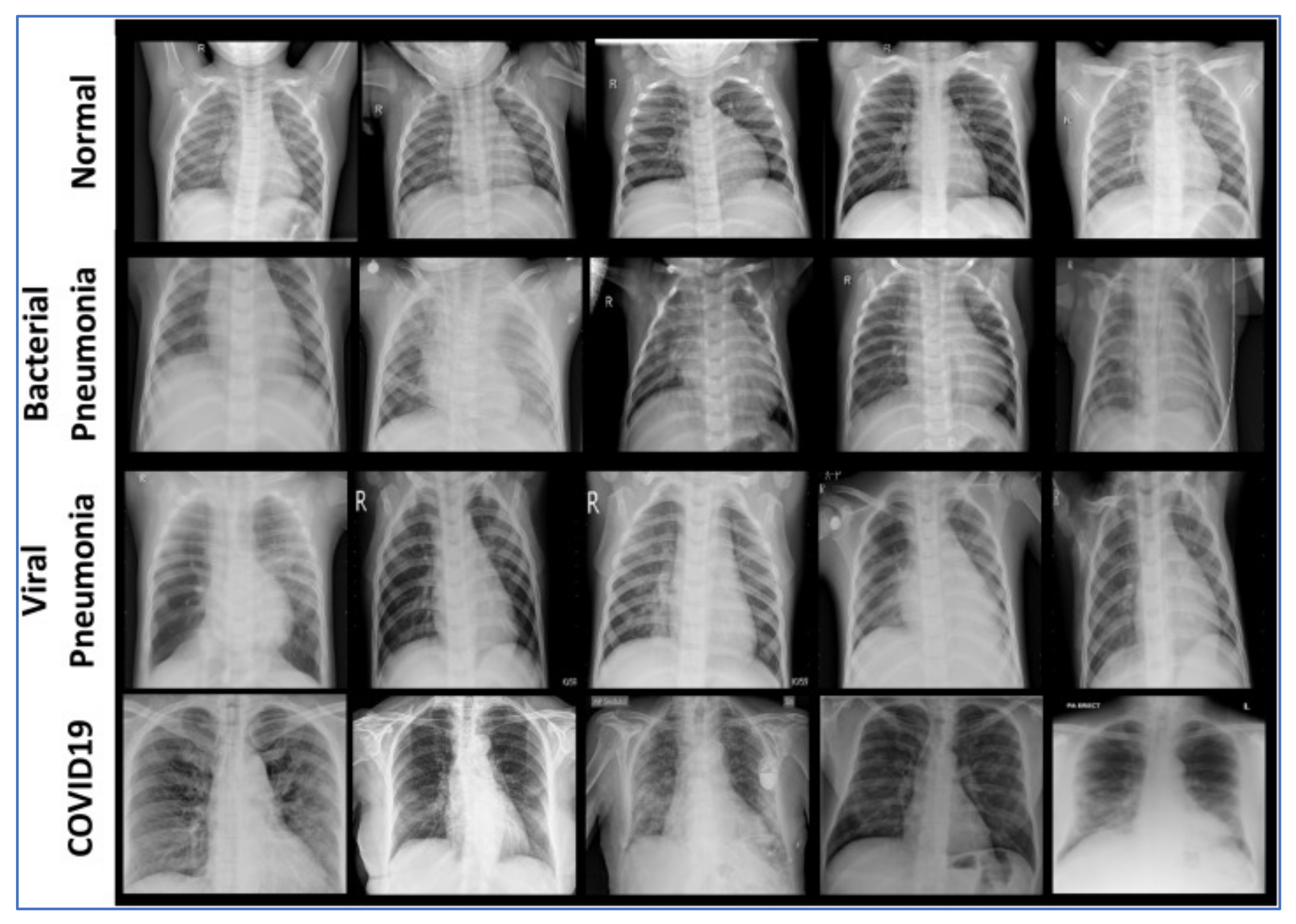
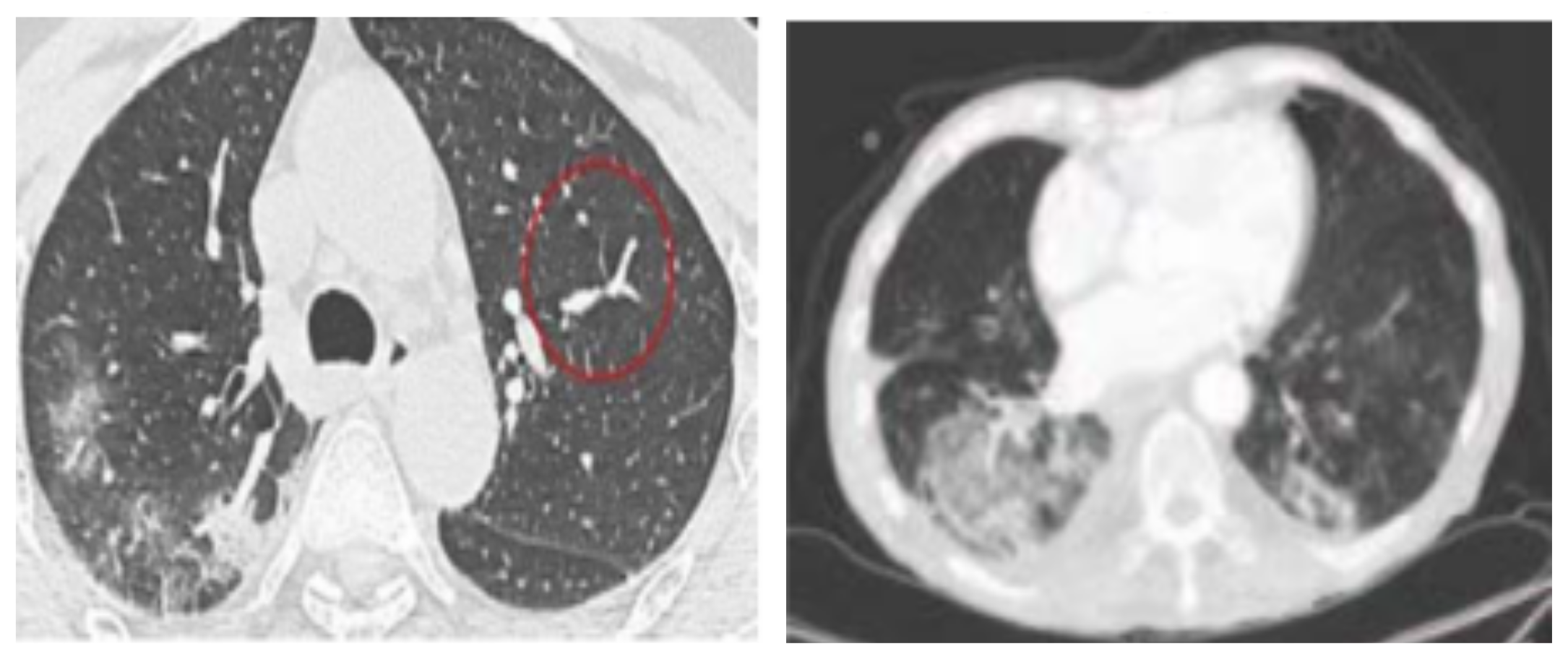
| Data/Class | Train | Validation | Test | Total |
|---|---|---|---|---|
| CXR data statistics. | ||||
| Non-COVID-19 | 70 | 20 | 18 | 108 |
| COVID-19 | 328 | 80 | 26 | 434 |
| Total | 398 | 100 | 44 | 542 |
| Augmented data statistics. | ||||
| Non-COVID-19 | 512 | 100 | 300 | 912 |
| COVID-19 | 512 | 100 | 300 | 912 |
| Kaggle pneumonia data statistics. | ||||
| Normal | 1341 | 8 | 234 | 1583 |
| Pneumonia | 3875 | 8 | 390 | 4273 |
| Total | 5216 | 16 | 624 | 5856 |
| CT scan data statistics. | ||||
| Non-COVID | 146 | 15 | 34 | 195 |
| COVID-19 | 183 | 57 | 35 | 275 |
| Total | 329 | 72 | 69 | 470 |
| Approach/Metrics | Acc (%) | DR (%) | FPR (%) |
|---|---|---|---|
| GMM-ML | 82.11 | 81.02 | 0.18 |
| GMM-B | 83.44 | 82.14 | 0.17 |
| GMM-V | 83.40 | 82.09 | 0.17 |
| MM-ML | 85.22 | 83.76 | 0.16 |
| MM-B | 87.45 | 85.52 | 0.14 |
| MM-V | 87.21 | 85.43 | 0.14 |
| OnMM-ML | 85.11 | 83.51 | 0.16 |
| OnMM-B | 87.33 | 85.02 | 0.14 |
| OnMM-V | 87.03 | 85.09 | 0.14 |
| inGMM-B | 83.42 | 82.11 | 0.17 |
| inMM-B | 87.34 | 85.01 | 0.14 |
| inMM-V | 87.01 | 85.06 | 0.14 |
| Approach/Metrics | Acc (%) | DR (%) | FPR (%) |
|---|---|---|---|
| GMM-ML | 87.66 | 85.80 | 0.13 |
| GMM-B | 88.90 | 86.98 | 0.11 |
| GMM-V | 88.12 | 86.41 | 0.12 |
| MM-ML | 90.54 | 88.54 | 0.10 |
| MM-B | 92.67 | 90.04 | 0.08 |
| MM-V | 92.61 | 90.01 | 0.08 |
| OnMM-ML | 90.03 | 88.12 | 0.10 |
| OnMM-B | 92.12 | 89.88 | 0.08 |
| OnMM-V | 92.06 | 89.81 | 0.10 |
| inGMM-B | 88.91 | 86.96 | 0.11 |
| inMM-B | 92.69 | 90.06 | 0.08 |
| inMM-V | 92.62 | 90.03 | 0.08 |
| Approach/Metrics | Acc (%) | DR (%) | FPR (%) |
|---|---|---|---|
| GMM-ML | 85.13 | 83.99 | 0.14 |
| GMM-B | 86.77 | 84.08 | 0.13 |
| GMM-V | 86.84 | 84.93 | 0.13 |
| MM-ML | 90.24 | 89.14 | 0.10 |
| MM-B | 91.95 | 93.44 | 0.09 |
| MM-V | 91.66 | 89.91 | 0.08 |
| OnMM-ML | 90.08 | 88.33 | 0.09 |
| OnMM-B | 90.32 | 89.17 | 0.09 |
| OnMM-V | 90.24 | 89.20 | 0.09 |
| inGMM-B | 86.78 | 84.09 | 0.13 |
| inMM-B | 90.31 | 89.14 | 0.09 |
| inMM-V | 90.21 | 89.20 | 0.09 |
| Approach/Metrics | Acc (%) | DR (%) | FPR (%) |
|---|---|---|---|
| GMM-ML | 78.45 | 76.35 | 0.22 |
| GMM-B | 79.11 | 77.70 | 0.20 |
| GMM-V | 79.32 | 76.74 | 0.20 |
| MM-ML | 81.05 | 80.71 | 0.20 |
| MM-B | 82.88 | 81.13 | 0.20 |
| MM-V | 82.77 | 81.33 | 0.18 |
| OnMM-ML | 80.66 | 88.55 | 0.20 |
| OnMM-B | 81.11 | 79.21 | 0.19 |
| OnMM-V | 81.06 | 79.53 | 0.19 |
| inGMM-B | 79.10 | 77.67 | 0.20 |
| inMM-B | 81.10 | 79.19 | 0.19 |
| inMM-V | 81.05 | 79.51 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallay, H.; Bourouis, S.; Bouguila, N. Online Learning of Finite and Infinite Gamma Mixture Models for COVID-19 Detection in Medical Images. Computers 2021, 10, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/computers10010006
Sallay H, Bourouis S, Bouguila N. Online Learning of Finite and Infinite Gamma Mixture Models for COVID-19 Detection in Medical Images. Computers. 2021; 10(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/computers10010006
Chicago/Turabian StyleSallay, Hassen, Sami Bourouis, and Nizar Bouguila. 2021. "Online Learning of Finite and Infinite Gamma Mixture Models for COVID-19 Detection in Medical Images" Computers 10, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/computers10010006





