Challenges and Opportunities in Identifying and Characterising Keratinases for Value-Added Peptide Production
Abstract
:1. Introduction
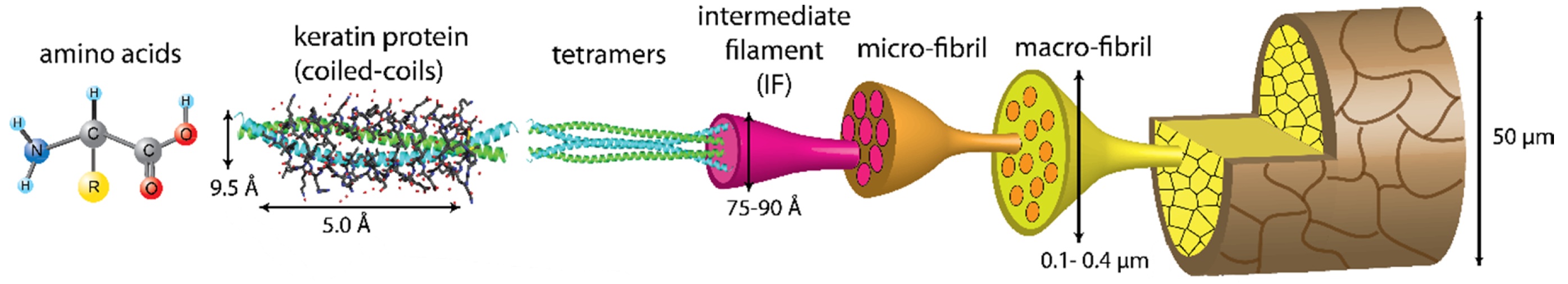
2. Keratin: A Complex and Strong Structure
3. Thermochemical Methods of Keratin Degradation
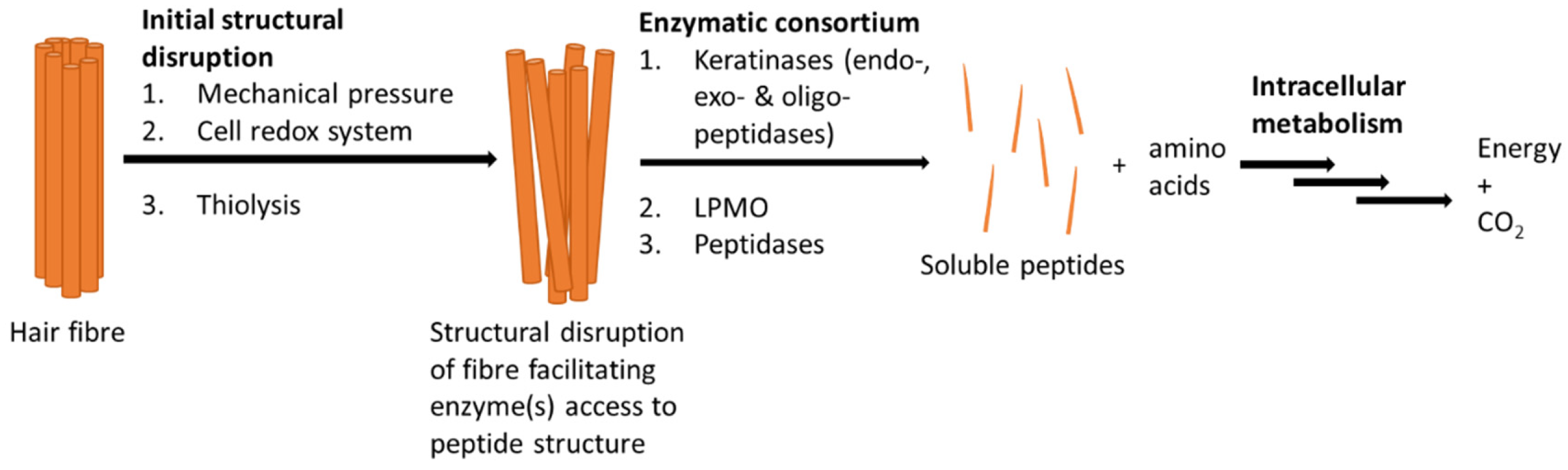
4. Microbial Degradation of Keratin
5. Characterisation and Comparison of Keratinases from S1, S8 and M4 Peptidase Families
5.1. S1, S8 and M4 Peptidase Families
5.2. Problems Associated with Keratinase Assays
5.3. The Effect of Additives on Selected S1, S8 and M4 Keratinases
5.4. Substrate Specificity
6. Potential Applications of Keratinases
7. Discovery and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of chicken feathers: A review on recycling and recovery route—current status and future prospects. Clean Technol. Environ. Policy 2017, 19, 2363–2368. [Google Scholar] [CrossRef]
- Thyagarajan, D.; Barathi, M.; Sakthivadivu, R. Scope of poultry waste utilization. IOSR-JAVS 2013, 6, 29–35. [Google Scholar]
- Gooding, C.H.; Meeker, D.L. Review: Comparison of 3 alternatives for large-scale processing of animal carcasses and meat by-products. PAS 2016, 32, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Sinkiewicz, I.; Staroszczyk, H.; Sliwinska, A. Solubilization of keratins and functional properties of their isolates and hydrolysates. J. Food Biochem. 2018, 42, e12494. [Google Scholar] [CrossRef]
- Chojnacka, K.; Gorecka, H.; Michalak, I.M.; Gorecki, H. A review: Valorization of keratinous materials. Waste Biomass Valoriz. 2011, 2, 3017–3021. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Zhang, J.; Du, G.; Chen, J. Keratin waste recycling based on microbial degradation: Mechanisms and prospects. ACS Sustain. Chem. Eng. 2019, 7, 9727–9736. [Google Scholar] [CrossRef]
- Jones, L.N. Hair structure anatomy and comparative anatomy. Clin. Dermatol. 2001, 19, 95–103. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberg, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Plowman, J.E. Proteomic database of wool components. J. Chromatog. B. 2003, 787, 63–76. [Google Scholar] [CrossRef]
- Da Silva, R.R. Different processes for keratin degradation: The ways for the biotechnological application of keratinases. J. Agric. Food Chem. 2018, 66, 9377–9378. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Y.; Rheinstädter, M.C. The structure of people’s hair. PeerJ 2014, 2, e619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: structure, mechanical properties, occurrence in biological organisms, and efforts of bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef] [Green Version]
- Ganske, F.; Meyer, H.H.; Deutz, H.; Bornscheuer, U. Enzyme-catalysed hydrolysis of 18-methyleicosanoic acid-cysteine thioester. Eur. J. Lipid Sci. Technol. 2003, 105, 627–632. [Google Scholar] [CrossRef]
- Koepke, V.; Nilssen, B. Wool surface properties and their influence on dye uptake—A microscopical study. J. Text. Inst. 1960, 51, T1398–T1413. [Google Scholar] [CrossRef]
- Wolski, T. Modified Keratin Proteins, Their Physiochemical Properties, Analysis and Application; Medical Academy: Lublin, Poland, 1985. [Google Scholar]
- Xie, H.; Li, S.; Zhang, S. Ionic liquids as novel solvents for the dissolution of and blending of wool keratin fibers. Green Chem. 2005, 7, 606–608. [Google Scholar] [CrossRef]
- Ningthoujam, D.S.; Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S. Keratinaceous Wastes and Their Valorization Through Keratinolytic Microorganisms. In Keratin; Blumenberg, M., Ed.; IntechOpen: London, UK, 2018; Volume 1, pp. 129–148. [Google Scholar]
- Wu, G. Amino Acids: Biochemistry and Nutrition, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Papadopoulis, M.C. Effect of processing on high-protein feedstuffs: a review. Biol. Wastes 1989, 29, 123–138. [Google Scholar] [CrossRef]
- Latshaw, J.D.; Musharaf, N.; Retrum, R. Processing of feather meal to maximize its nutritional value for poultry. Animal Feed Sci. Technol. 1994, 47, 179–188. [Google Scholar] [CrossRef]
- Schwass, D.E.; Finley, J.W. Heat and alkaline damage to proteins: Racemization and lysinoalanine formation. J. Ag. Food Chem. 1984, 32, 1377–1382. [Google Scholar] [CrossRef]
- Liardon, R.; Hurrell, R.F. Amino acid racemization in heated and alkali-treated proteins. J. Ag. Food Chem. 1983, 31, 432–437. [Google Scholar] [CrossRef]
- Liardon, R.; Ledermann, S. Racemization kinetics of free and protein-bound amino acids under moderate alkaline treatment. J. Agric. Food Chem. 1986, 34, 557–565. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, K.; Yamauchi, A.; Kusonoki, T.; Kohda, A.; Konishi, Y. Preparation of stable aqueous solution of keratins, and physiochemical and degradational properties of films. J. Biomed. Mater. Res. Part Res. 1996, 31, 439–444. [Google Scholar] [CrossRef]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef]
- Tonin, C.; Aluigi, A.; Vineis, C.; Varesano, A.; Montarsolo, A.; Ferrero, F. Thermal and structural characterizationof poly (ethylene-oxide)/keratin blend films. J. Therm. Anal. Calorim. 2007, 89, 601–608. [Google Scholar] [CrossRef]
- Wang, K.; Li, R.; Ma, J.H.; Jian, Y.K.; Che, J.N. Extracting keratin from wool using cysteine. Green Chem. 2016, 18, 476–481. [Google Scholar] [CrossRef]
- Torchinski, Y.M. Sulfur in Proteins; Pergamon Press: Oxford, UK, 1981. [Google Scholar]
- Cardamone, J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR). J. Mol. Struct. 2010, 969, 97–105. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; Van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef]
- Cardamone, J.M.; Nunez, A.; Garcia, R.A.; Aldema-Ramos, M. Characterizing wool keratin. Lett. Mater. Sci. 2009, 2009, 147175. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.J.; Worth, G.H.; Roddick-Lanzilotta, A.D.; Rankin, D.A.; Ellis, G.D.; Mesman, P.J.R.; Summers, C.G.; Singleton, D.J. The Production of Soluble Keratin Derivatives. U.S. 7148327B2, 2001. [Google Scholar]
- Cornfield, M.C.; Robson, A. The amino acid composition of wool. Biochem. J. 1955, 59, 62–68. [Google Scholar]
- Mellet, P. The influence of alkali treatment on native and denatured proteins. Tex. Res. J. 1968, 38, 977–983. [Google Scholar] [CrossRef]
- Masters, P.M.; Friedman, M. Racemization of amino acids in alkali-treated food proteins. J. Agric. Food Chem. 1979, 27, 507–511. [Google Scholar] [CrossRef]
- Provansal, M.M.P.; Cug, J.L.A.; Cheftal, J.C. Chemical and nutritional modifications of sunfower proteins due to alkaline processing. Formation of amino acid cross-links and isomerization of lysine residues. J. Agric. Food Chem. 1975, 23, 938–943. [Google Scholar] [CrossRef]
- Gaidau, C.; Epure, D.-G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.C.W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration—an ecological approach. J. Clean. Prod. 2019, 236, 117586. [Google Scholar] [CrossRef]
- Koleva, M.; Danalev, D.; Ivanova, D.; Vezenkov, L.; Vassiliev, N. Synthesis of two peptide mimetics as markers for chemical changes of wool’s keratin during skin unhairing process and comparison the wool quality obtaianed by ecological methods for skin unhairing. Bulg. Chem. Commun. 2009, 41, 160–164. [Google Scholar]
- Banga, A.K. Therapeutic Peptides and Proteins: Formulation, Process and Development Systems; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Murray, K.; Rasmussen, P.; Neustaedter, J.; Luck, J.M. The hydrolysis of arginine. J. Biol. Chem. 1965, 240, 705–709. [Google Scholar]
- Daft, F.S.; Coghill, R.D. The alkaline decomposition of serine. J. Biol. Chem. 1931, 90, 341–350. [Google Scholar]
- Hunt, S. The non-protein amino acids. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Springer: Dordrecht, Germany, 1985. [Google Scholar]
- Stapleton, I.; Swan, J. Amino acids and peptides. VI. Studies on cystine and αα’-dimethylcystine in relation to the alkaline degradation of protein disulphides. Aust. J. Chem. 1960, 13, 416–425. [Google Scholar] [CrossRef]
- Ohta, T.; Suzuki, S.; Todo, M.; Kurechi, T. The decomposition of tryptophan in acid solutions: specific effect of hydrochloric acid. Chem. Pharm. Bull. 1981, 29, 1767–1771. [Google Scholar] [CrossRef] [Green Version]
- Tung, W.S.; Daoud, W.A. Photocatalytic self-cleaning keratins: A feasibility study. Acta Biomater. 2009, 5, 50–56. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Kurbanoglu, N.I. A new process for the utilization of ram horn waste. J. Biosci. Bioeng. 2002, 94, 202–206. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, J.; Zhao, Z.; Liu, X.; Li, Z.; Han, Y.; Hu, J. Isolation and characterization of biofunctional keratin particles extracted from wool waste. Powder Technol. 2013, 246, 356–362. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Kurbanoglu, N.I. Ram horn hydrolysate as enhancer of xanthan production in batch culture of Xanthomonas campestris EBK-4 isolate. Process Biochem. 2007, 42, 1146–1149. [Google Scholar] [CrossRef]
- Brandelli, A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioproc. Technol. 2008, 1, 105–116. [Google Scholar] [CrossRef]
- Sharma, R.; Devi, S. Versatility and commercial status of microbial keratinases: A review. Rev. Environ. Sci. Biotechnol. 2018, 17, 19–45. [Google Scholar] [CrossRef]
- Muhsin, T.H.; Aubaid, A.H. Partial purification and some biochemical characteristics of exocellular keratinase from Trichophyton mentagrophytes var erinacei. Mycopathologia 2001, 150, 121–125. [Google Scholar] [CrossRef]
- Rai, S.K.; Mukherjee, A.K. Optimization of the production of an oxidant and detergent-stable alkaline beta-keratinase from Brevibacillus sp. strain AS-S10-11: application of enzyme in laundry detergent formulations and in the leather industry. Biochem. Eng. J. 2011, 54, 47–56. [Google Scholar] [CrossRef]
- Thankaswamy, S.R.; Sundaramoorthy, S.; Palanivel, S.; Ramudu, K.N. Improved microbial degradation of animal hair waste from leather industry using Brevibacterium luteolum (MTCC 5982). J. Clean. Prod. 2018, 189, 701–708. [Google Scholar] [CrossRef]
- Nam, G.-W.; Lee, D.-W.; Lee, H.-S.; Lee, N.-J.; Kim, B.-C.; Choe, E.-A.; Hwang, J.-K.; Suhartono, M.T.; Pyun, Y.-R. Native feather-degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch. Microbiol. 2002, 178, 538–547. [Google Scholar] [CrossRef]
- Kshteri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Sailo, B.; Singh, S. Feather degrading, phytostimulating, and biocontrol potential of actinobacteria from North Easatern Himalayan Region. J. Basic Microbiol. 2018, 58, 730–738. [Google Scholar] [CrossRef]
- Mamangkey, J.; Suryanto, D.; Munir, E.; Mustopa, A.Z. Isolation, molecular identification and verification of gene encoding bacterial keratinase from crocodile (crocodylus porosus) feces. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012085. [Google Scholar] [CrossRef]
- Pernicova, I.; Enev, V.; Marova, I.; Obruca, S. Interconnection oof waste chicken feather biodegradation and keratinase and mcl-PHA prooduction employing Pseudomonas putida KT2440. Appl. Food Biotechnol. 2019, 6, 83–90. [Google Scholar]
- Fuke, P.; Gujar, V.V.; Khardenavis, A. Genome annotation and vlidation of keratin hydrolyzing proteolytic enzymes from Serratia marcescens EGD-HP20. Appl. Biochem. Biotechnol. 2018, 18, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Fuke, P.; Pal, R.R.; Khardenavis, A.A.; Purohit, H. In silico characterization of broad range proteases produced by Serratia marcescens EGD-HP20. J. Basic Microbiol. 2018, 58, 492–500. [Google Scholar] [CrossRef]
- Bhari, R.; Kaur, M.; Singh, R.S. Thermostable and halotolerant keratinae from Bacillus aerius NSMk2 with remarkable dehairing and laundry applications. J. Basic Microbiol. 2019, 59, 555–558. [Google Scholar] [CrossRef]
- Pawar, V.A.; Prajapati, A.S.; Akhani, R.C.; Patel, D.H.; Subramanian, R.B. Molecular and biochemical characteriztion of a thermostable keratinase from Bacillus altitudinis RBDV1. 3 Biotech 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Cao, S.; Li, D.; Ma, X.X.Q.; Song, J.; Lu, F.; Li, Y. A novel unhairing enzyme produced by heterolgous expression of keratinase gene (kerT) in Bacillus subtilis. World J. Microbiol. Biotechnol. 2019, 35, 122–131. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatia, D.; Khatak, S.; Kumar, R.; Sharma, A.; Malik, D.K. Optimization and purification of keratinase from Bacillus anthracis with dehairing application. J. Pure Appl. Microbiol. 2019, 13, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Kalaikumari, S.S.; Vennila, T.; Monika, V.; Chandraraj, K.; Gunasekaran, P. Bioutilization of poultry feather for keratinase production and its application in leather industry. J. Clean. Prod. 2019, 208, 44–45. [Google Scholar] [CrossRef]
- Hamiche, S.; Mechri, S.; Khelouia, L.; Annane, R.; El Hattab, M.; Badis, A.; Jaouadi, B. Purification and biochemical characterization of two keratinases from Bacillus amyloliquefaciens S13 isolated from marine brown algae Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int. J. Biol. Macromol. 2019, 122, 758–769. [Google Scholar] [CrossRef]
- Devi, C.S.; Shankar, R.S.; Kumar, S.; Mohanasrinivasan, B. Production of keratinase from a newly isolates feather degrading Bacillus cereus VITSDVM4 from poultry waste. Natl. Acad. Sci. Lett. 2018, 41, 307–311. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Varghese, R.; Ahmed, B.A.; Duraipandiyan, V.; Al-Dhabi, N.A. Optimizing the fermentation conditions and enhanced production of keratinase from Bacillus cereus isolated from halophilic environment. Saudi J. Biol. Sci. 2019, 26, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, A.M.; El-Gamal, M.S.; Ismail, S.A.; Emran, M.A. Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1. JGEB 2018, 16, 311–318. [Google Scholar] [CrossRef]
- Cavello, I.; Urbieta, M.S.; Segretin, A.B.; Giaveno, A.; Cavalitto, S.; Donati, E.R. Assessment of keratinase and other hydrolytc enzymes in thermophilic bacteria isolated from geothermal areas in Patagonia Argentina. Geomicrobiol. J. 2018, 35, 156–165. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Adejumo, I.O. Efficacy of crude and immobilized enzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment. Environ. Technol. Inno. 2018, 11, 116–124. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abdel-Fattah, A.M.; Ismail, S.A.; El-Gamal, M.S.; Esawy, M.A.; Emran, M.A. Optimization, characterization and thermodynamic studies on B. licheniformis ALW1 keratinase. Egypt J. Chem. 2018, 61, 591–607. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Sun, R.; Tang, Z.; Bu, T.; Wu, Q.; Li, C.; Chen, H. Biodegradation of feather waste keratin by keratin-degrading strain Bacillus subtilis 8. J. Microbiol. Biotechnol. 2018, 28, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Oluwaseun, A.C.; Phazang, P.; Sarin, N.B. Production of ecofriendly biofertilizers produced from crude and immobilized enzymes from Bacillus subtilis CH008 and their effect on the growth of Solanum lycopersicum. Plant Arch. 2018, 18, 1455–1462. [Google Scholar]
- Nagarajan, S.; Eswaran, P.; Masilamani, R.P.; Natarajan, H. Chicken feather compost to promote the plant growth activity by using keratinolytic bacteria. Waste Biomass Valor. 2018, 9, 531–538. [Google Scholar] [CrossRef]
- Imtiaz, A.; Rehman, A. Bacillus subtilis BML5 isolated from soil contaminated with poultry waste has keratinolytic activity. Pakistan J. Zool. 2018, 50, 143–148. [Google Scholar] [CrossRef]
- De Paiva, D.P.; De Oliveira, S.S.A.; Mazotto, A.M.; Vermehlo, A.B.; De Oliveira, S.S. Keratinolytic activity of Bacillus subtilis LFB-FIOCRUZ 1266 enhanced by whole-cell mutagenesis. 3 Biotech 2019, 9, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Prasad, R.K.; Chatterjee, S.; Sharma, A.; Variable, M.G.; Yadav, K.K. Characterization of Bacillus species with keratinase and cellulase properties isolated from feather dumping and cockroach gut. Proc. Natl. Acad. Sci. India. Sect. B Biol. Sci 2019, 89, 1079–1086. [Google Scholar] [CrossRef]
- Suharti, D.R.T.; Nilamsari, N.R. Isolation and characterization of newly keratinase producing Bacillus sp. N1 from tofu liquid waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012088. [Google Scholar] [CrossRef]
- Jin, M.; Chen, C.; He, X.; Zeng, R. Characterization of an extreme alkaline-stable keratinase from the draft genome of feather-degrading Bacillus sp. JM7 from deep-sea. Acta Oceanol. Sin. 2019, 38, 87–95. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Z.; Long, X.; Tian, Y.; Shi, B. High-expression keratinase by Bacillus subtilis SCK6 for enzymatic dehairing of goatskins. Int. J. Biol. Macromol. 2019, 135, 119–126. [Google Scholar] [CrossRef]
- Koentjoro, M.P.; Prasetyo, E.N.; Rahmatullah, A.M. Optimization of keratinase production by Bacillus SLII-I bacteria in chicken feather waste medium. ARPN J. Engin. Appl. Sci. 2018, 13, 482–488. [Google Scholar]
- Nurkhasanah, U.; Suharti. Preliminary study on keratinase fermentation by Bacillus sp. MD24 under solid state fermentation. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012016. [Google Scholar] [CrossRef] [Green Version]
- Suharti, S.; Riesmi, M.T.; Hidayati, A.; Zuhriyah, U.F.; Wonorahardjo, S.; Susanti, E. Enzymatic dehairing of goat skin using keratinase from Bacillus sp. MD24, a newly isolated soil bacterium. Pertanika J. Trop. Agric. Sci. 2018, 41, 1449–1461. [Google Scholar]
- Ashokkumar, M.; Irudayaraj, G.; Yellapu, N.; Manonmani, A.M. Molecular characterization of bmyC gene of the mosquito pupicidal bacterial, Bacillus amyloliquefaciens (VCRC B483) and in silico analysis of bacillomycin D snthetase C protein. World J. Microbiol. Biotechnol. 2018, 34, 116–126. [Google Scholar] [CrossRef]
- Yusuf, I.; Ahmad, S.A.; Pang, L.Y.; Yasid, N.A.; Shukor, M.Y. Effective production of keratinase by gellum gum-immobilised Alcaligenes sp. AQ05-001 using heavy metal-free and polluted feather wastes as substrates. 3 Biotech 2019, 9, 32–43. [Google Scholar] [CrossRef]
- Sultana, N.; Saha, P. Studies on potential application of crude keratinase enzyme from Stenotrophomonas sp. for dehairing in leather processing industry. J. Environ. Biol. 2018, 39, 324–330. [Google Scholar] [CrossRef]
- Kang, D.; Herschend, J.; Al-Soud, W.A.; Mortensen, M.S.; Gonzalo, M.; Jacquiod, S.; Sorensen, S.J. Enrichment and characterization of an environmental microbial consortium displaying efficient keratinolytic acitivty. Bioresour. Technol. 2018, 270, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermophytes. Med. Mycol. J. 2019, 57, 13–22. [Google Scholar] [CrossRef]
- Pillai, P.; Mandge, S.; Archana, G. Statistical optimization of production and tannery application of a keratinolytic serine protease from Bacillus subtilis P13. Process Biochem. 2011, 46, 1110–1117. [Google Scholar] [CrossRef]
- Purchase, D. Microbial Keratinases: Characteristics, Biotechnological Applications and Potential; CABI: Wallingford, UK, 2016. [Google Scholar]
- Gupta, R.; Sharma, R.; Beg, Q.K. Revisiting microbial keratinases: Next generation proteases for sustainable biotechnology. Crit. Revs. Biotechnol. 2013, 33, 216–228. [Google Scholar] [CrossRef]
- Elhoul, M.B.; Jaouadi, N.Z.; Rekik, H.; Benmrad, M.O.; Mechri, S.; Moujehed, E.; Kourdali, S.; Hattab, M.E.; Badis, A.; Bejar, S.; et al. Biochemical and molecular characterization of new keratinoytic protease from Actinomadura viridilutea DZ50. Int. J. Biol. Macromol. 2016, 92, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Habbeche, A.; Saoudi, B.; Jaouadi, B.; Haberra, S.; Kerouaz, B.; Boudelaa, M.; Badis, A.; Ladjama, A. Purification and biochemical characterization of a detergent-stable keratinase from a newly thermophilic actinomycete Actinomadura keratinilytica strain Cpt29 isolated from poultry compost. J. Biosci. Bioeng. 2014, 117, 413–421. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Kanoun, S.; Manni, L.; Nasri, M. Production and biochemical and molecular characterization of a keratinolytic serine protease from chicken feather-degrading Bacillus licheniformis RPk. Can. J. Microbiol. 2009, 55, 427–436. [Google Scholar] [CrossRef]
- Fakhfakh-Zouari, N.; Hmidet, N.; Haddar, A.; Kanoun, S.; Nasri, M. A novel serine metalloprotease from a newly isolated Bacillus pumilus A1 grown on chicken feather meal: biochemical and molecular characterization. Appl. Biochem. Biotechnol. 2010, 162, 329–344. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y.; Cao, Y.; Huang, Y.; Chang, Z.; Huang, H. Production and characterization of keratinolytic proteases by a chicken feather-degrading thermophilic strain, Thermoactinomyces sp. YT06. J. Microbiol. Biotechnol. 2017, 27, 2190–2198. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shi, P.-J.; Han, X.-Y.; Meng, K.; Yang, P.-L.; Wang, Y.-R.; Luo, H.-Y.; Wu, N.-F.; Yao, B.; Fan, Y.-L. Functional expression of the keratinolytic serine protease gene sfp2 from Streptomyces fradiae var. k11 in Pichia pastoris. Protein Expres. Purif. 2007, 54, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Benkiar, A.; Nadia, Z.J.; Badis, A.; Rebzani, F.; Soraya, B.T.; Rekik, H.; Naili, B.; Ferradji, F.Z.; Bejar, S.; Jaouadi, B. Biochemical and molecular characterization of a thermo- and detergent-stable alkaline serin keratinolytic protease from Bacillus circulans strain DZ100 for detergent formulations and feather-biodegradation process. Int. Biodeterior. Biodegradation 2013, 83, 129–138. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Jiang, L.; Du, G.; Chen, J. Cloning, heterologous expression and characterization of two keratinases from Stenotrophomonas maltophilia BBE11-1. Proc. Biochem. 2014, 49, 647–654. [Google Scholar] [CrossRef]
- Jaouadi, B.; Ellouz-Chaabouni, S.; Rhimi, M.; Bejar, S. Biochemical and molecular characterization of a detergent-stable serine alkaline protease from Bacillus pumilus CBS with high catalytic efficiency. Biochimie 2008, 90, 1291–1305. [Google Scholar] [CrossRef]
- Rajput, R.; Sharma, R.; Gupta, R. Cloning and characterization of a thermostable detergent-compatible recombinant keratinase from Bacillus pumilus KS12. IUBMB 2011, 58, 109–118. [Google Scholar]
- Jaouadi, N.Z.; Rekik, H.; Elhoul, M.B.; Rahem, F.Z.; Hila, C.G.; Aicha, H.S.B.; Badis, A.; Toumi, A.; Bejar, S.; Jaouadi, B. A novel keratinase from Bacillus tequilensis strain Q7 with promising potential for the leather bating process. Int. J. Biol. Macromol. 2015, 79, 952–964. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakrabarti, K.; Chattopadhyay, D. Cloning of feather-degrading minor extracellular protease from Bacillus cereus DCUW: dissection of the structural domains. Microbiology 2009, 155, 2049–2057. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cheng, G.; Ren, Y.; Dai, Z.; Zhao, Z.-S.; Liu, F.; Li, S.; Wei, Y.; Xiong, J.; Tang, X.-F.; et al. Degradation of intact chicken feathers by Thermoactinomyces sp. CDF and characterization of its keratinolytic protease. Appl Microbiol. Biotechnol. 2015, 99, 3949–3959. [Google Scholar] [CrossRef]
- Wu, W.-L.; Chen, M.-Y.; Tu, I.-F.; Lin, Y.-C.; Kumar, N.E.; Chen, M.-Y.; Ho, M.-C.; Wu, S.-H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 4658–4669. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Bian, Y.; Tang, X.-F.; Xiao, G.; Tang, B. Enhancement of keratinolytic activity of a thermophilic subtilase by improving its autolysis resistance and thermostability under reducing conditions. Appl. Microbiol. Biotechnol. 2010, 87, 999–1006. [Google Scholar] [CrossRef]
- Gegeckas, A.; Gudiukaite, R.; Debski, J.; Citavicius, D. Keratinous waste decomposition and peptide production by keratinase from Geobacillus stearothermophilus AD-11. Int. J. Biol. Macromol. 2015, 75, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Murty, N.A.R.; Gupta, R. Molecular characterization of N-terminal pro-sequence of keratinase ker P from Pseudomonas aeruginosa: identification of region with chaperone activity. Appl. Biochem. Biotechnol. 2011, 165, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Mitsuiki, S.; Ichikawa, M.; Oka, T.; Sakai, M.; Moriyama, Y.; Sameshima, Y.; Goto, M.; Furukawa, K. Molecular characterization of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Enzyme Microb. Technol. 2004, 34, 482–489. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakrabarti, K. Degradtion of raw feather by a novel high molecular weight extracellular protease from newly isolated Bacillus cereus DCUW. J. Ind. Microbiol. Biotechnol. 2008, 35, 825–834. [Google Scholar] [CrossRef]
- Tripathi, L.P.; Sowdhamini, R. Genome-wide survey of prokaryotic serine proteases: analysis of distribution and domain architectures of five serine protease families in prokaryotes. BMC Genomics 2008, 9, 549–576. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [Green Version]
- Siezen, R.J. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991, 4, 719–737. [Google Scholar] [CrossRef]
- Page, M.J.; Di Cera, E. Serine peptidases: classification, structure and function. Cell Mol. Life Sci. 2008, 65, 1220–1236. [Google Scholar] [CrossRef]
- Donlon, J.; Polaina, J.; MacCabe, A.P. (Eds.) Industrial Enzymes; Springer: Dordrecht, Germany, 2007. [Google Scholar]
- Jongeneel, C.V.; Bouvier, J.; Bairoch, A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989, 242, 211–214. [Google Scholar] [CrossRef]
- De Kreij, A.; Venema, G.; Van den Burg, B. Substrate specificity in the highly heterogeneous M4 peptidase family is determined by a small subset of amino acids. JBC 2000, 275, 31115–31120. [Google Scholar] [CrossRef] [Green Version]
- Gregg, K.A. A comparison of genomic coding sequences for feather and scale keratins: Structural and evolutionary implications. EMBO J. 1984, 3, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Gradisar, H.; Friedrich, J.; Krizaj, I.; Jerala, R. Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl. Environ. Microbiol. 2005, 71, 3420–3426. [Google Scholar] [CrossRef] [Green Version]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Di Cera, E. Serine proteases. IUBMB Life 2009, 61, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Chen, X.L. Extracellular metalloproteases from bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 253–262. [Google Scholar] [CrossRef]
- Wainwright, M. A new method for determining the microbial degradation of keratin in soils. Experientia 1982, 38, 243–244. [Google Scholar] [CrossRef]
- Riffel, A.; Lucas, F.; Heeb, P.; Brandelli, A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch. Microbiol. 2003, 179, 258–265. [Google Scholar] [CrossRef]
- Navone, L.; Speight, R. Understanding the dynamics of keratin weakening and hydrolysis by proteases. PLoS ONE 2018, 13, e0202608. [Google Scholar] [CrossRef]
- Farag, A.M.; Hassan, M.A. Purification, characterization and immobilization of a keratinase from Aspergillus orizae. Enzyme Microb. Technol. 2004, 34, 85–93. [Google Scholar] [CrossRef]
- Grazziotin, A.; Pimentel, F.A.; De Jong, E.V.; Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Animal Feed Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Gill, S.C.; Von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Daroit, D.J.; Corrêa, A.P.F.; Brandelli, A. Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractus mesopotamicus. Int. Biodeter. Biodegr. 2009, 63, 358–363. [Google Scholar] [CrossRef]
- Pereira, J.Q.; Lopes, F.C.; Petry, M.V.; Da Costa Medina, L.F.; Brandelli, A. Isolation of three novel Antarctic psychrotolerant feather-degrading bacteria and partial purification of keratinolytic enzyme from Lysobacter sp. A03. Int. Biodeter. Biodegr. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Wawrzkiewicz, K.; Wolski, T.; Lobarzewski, J. Screening the keratinoltic activity of dermatophytes in vitro. Mycopathologia 1991, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bressollier, P.; Letourneau, F.; Urdaci, M.; Verneuil, B. Purification and characterization of a keratinolytic serine proteinase from Streptomyces albidoflavus. Appl. Environ. Microbiol. 1999, 65, 2570–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.J.; Yoshimura, E.; Sakai, H.; Wakagi, T.; Matsuzawa, H. Weakly bound calcium ions involved in the thermostability of aqualysin I, a heat-stable subtilisin-type protease of Thermus aquaticus YT-1. BBA-Protein Struct. M. 1999, 133, 132–138. [Google Scholar] [CrossRef]
- Meng, K.; Li, J.; Cao, Y.; Shi, P.; Wu, B.; Han, X.; Bai, Y.; Wu, N.-F.; Yao, B. Gene cloning and heterologous expression of a serine protease from Streptomyces fradiae var.k11. Can. J. Microbiol. 2007, 53, 186–195. [Google Scholar] [CrossRef]
- Öztürk, N.Ç.; Kazan, D.; Denizci, A.A.; Erarslan, A. The influence of copper on alkaline protease stability toward autolysis and thermal inactivation. Eng. Life Sci. 2012, 12, 662–671. [Google Scholar] [CrossRef]
- Walker, J.M. The Proteins Protocols Handbook, 2nd ed.; Humana Press: Totowa, NJ, USA, 1996. [Google Scholar]
- Fershi, A.R. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding; Freeman W. H. & Co.: New York, NY, USA, 1999. [Google Scholar]
- Kunugi, S.; Tanaka, N. Cold denaturation of proteins under high pressure. Biochim. Biophys. Acta 2002, 1595, 309–314. [Google Scholar] [CrossRef]
- Salvi, G.; De Los Rios, P.; Vendruscolo, M. Effective interactions between chaotropic agents and proteins. Proteins Struct. Funct. Bioinf. 2005, 61, 429–499. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. J. Biosci. Bioeng. 2006, 102, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.J.; Beys da Silva, W.O.; Termignoni, C. Properties of a non collagen-degrading Bacillus subtilis keratinase. Can. J. Microbiol. 2008, 54, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rajput, R.; Sharma, R.; Gupta, N. Biotechnological applications and prospective market of microbial keratinases. Appl. Microbiol. Biotechnol. 2013, 97, 9931–9940. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Speight, R.E. Enzyme systems for effective dag removal from cattle hides. Anim. Prod. Sci. 2019, 59, 1387–1398. [Google Scholar] [CrossRef] [Green Version]
- Alba, A.C.; Strauch, T.A.; Keisler, D.H.; Wells, K.D.; Kesler, D.C. Using a keratinase to degrade chicken feathers for improved extraction of glucocorticoids. Gen. Comp. Endocr. 2019, 270, 35–40. [Google Scholar] [CrossRef]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: enzymatic fibre spearation and recycling of wool/polyester fabric blends. J. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- El Boushy, A.R.Y.; Van der Poel, F.B. Poultry by-Products. In Handbook of Poultry Feed from Waste; Springer: Dordrecht, Germany, 2000; pp. 90–152. [Google Scholar] [CrossRef]
- Sundaram, M.; Legadavi, R.; Banu, N.A.; Gayathri, V.; Palanisammy, A. A study of antibacterial activity of keratin nanoparticles from chicken feather waste against Staphylococcus aureus (Bovine mastitis bacteria) and its antioxidant activity. Eur. J. Biotechnol. Biosci. 2015, 6, 1–5. [Google Scholar]
- Ohba, R.; Deguchi, T.; Kishikawa, M.; Arsyad, F.; Morimura, S.; Kida, K. Physiological function of enzymatic hydrolysates of collagen or keratin contained in livestock or fish waste. Food Sci. Technol. Res. 2003, 9, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, W.; Shi, B.-H.; Li, J.-C.; Zhang, R.-Z.; Liu, S.-T.; Chen, R.-M.; Gao, W.-H.; Chen, G.-R.; Zheng, Y.-Q.; et al. Anti-Inflammatory Activity of Antelope Horn Keratin and its Tryptic Hydrolysate; Nutrition Press Inc.: Trumbull, CT, USA, 1999. [Google Scholar]
- Kelly, R.; Ellis, G.; Macdonald, R.; McPherson, R.; Middlewood, P.; Nuthall, M.; Rao, G.-F.; Roddick-Lanzilotta, A.; Sigurjonsson, G.; Singleton, D. Keratin and Soluble Derivatives Thereof for a Nutraceutical and to Reduce Oxidative Stress and to Reduce Inflammation and to Promote Skin Health. U.S. Patent 0065506, 22 March 2007. [Google Scholar]
- Kelly, R.J.; Ellis, G.D.; Macdonald, R.J.; McPherson, R.A.; Middlewood, P.G.; Nuthall, M.G.; Rao, G.-F.; Roddick-Lanzilotta, A.D.; Sigurjonsson, G.F.; Singleton, D.J. Nutraceutical Composition Comprising Soluble Keratin or Derivatives Thereof. U.S. Patent 7579317, 25 August 2009. [Google Scholar]
- Cutler, P. Protein Purification Protocols; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Zeng, W.-C.; Zhang, W.-C.; Zhang, W.-H.; Shi, B. Antioxidant activity and characterization of bioactive polypeptides from bovine hair. Funct. Polym. 2013, 73, 573–578. [Google Scholar] [CrossRef]
- Jones, L.N.; Sinclair, R.D.; Ecroyd, H.; Lui, Y.; Bennett, L.E. Bioprospecting keratinous material. Int. J. Trichol. 2010, 2, 47–49. [Google Scholar] [CrossRef]
- Fontoura, R.; Daroit, D.J.; Correa, A.P.F.; Meira, S.M.M.; Mosquera, M.; Brandelli, A. Production of feather hydrolysates with antioxidant, angiotensin-1 converting enzyme- and dipeptidyl peptidase-IV-inhibitory activities. New Biotechnol. 2014, 31, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, I.; Lee, Y.-J.; Song, K.; Jin, H.-S.; Lee, J.-E.; Kim, D.; Lee, D.-W.; Kang, N.J. Low molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J. Biotechnol. 2018, 271, 17–25. [Google Scholar] [CrossRef]
- Jin, H.-S.; Song, K.; Baek, J.-H.; Lee, J.-E.; Kim, D.J.; Nam, G.-W.; Kang, N.J.; Lee, D.-L. Identification of matrix metalloproteinasae-1-suppressive peptides in feather hydrolysates. J. Agric. Food Chem. 2018, 66, 12719–12729. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-S.; Park, S.Y.; Kim, J.-Y.; Lee, J.-E.; Lee, H.-S.; Kang, N.J.; Lee, D.-W. Fluorescence-based quantification of bioactive keratin peptides from feathers for optimising large-scale anaerobic fermentation and purification. Biotechnol. Bioproc. Eng. 2019, 2, 240–249. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, A.; Furuta, Y.; Takesima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Tang, L.; Ollague, S.J.; Kelly, R.; Kirsner, R.S.; Li, J. Wool-derived keratin stimulates uman keratinocyte migration and types IV and VIIcollagen expression. Exp. Dermatol. 2012, 21, 458–460. [Google Scholar] [CrossRef]
- Loan, F.; Marsh, C.; Cassidy, S.; Simcock, J. Keratin-based products for effective wound care management in superficial and partial thickeness burns injuries. Burns 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Pack. Shelf Life 2017, 12, 100–110. [Google Scholar] [CrossRef]
| Amino Acid | Degradation Products | Reference |
|---|---|---|
| Asparagine | Aspartate, ammonia | [41] |
| Glutamine | Glutamate, ammonia | [41] |
| Arginine | Ornithine, citrulline, 3-aminopiperidin-2-one | [42] |
| Serine | Glycine, alanine, oxalic acid, lactic acid, ammonia | [43] |
| Threonine | Glycine, alanine, α-aminobutyric acid, ammonia | [44] |
| Cysteine | Pyruvic acid, sodium sulfide, ammonia | [45] |
| * Cystine, lysine, arginine | Lanthionine, lysinoalanine, ornithinalanine; * dehydroalanine [39] | [35,36] |
| L-amino acids | D-amino acids | [37] |
| Amino Acid | Degradation Products | Reference |
|---|---|---|
| Asparagine | Aspartate, ammonia | [19,41] |
| Glutamine | Glutamate, ammonia | [19,41] |
| Methionine | Methionine sulfoxide | [19] |
| Tryptophan | Oxindolylalanine, dioxindolylalanine | [46] |
| Organism | Strain | Keratinase Name | Accession No. 1 | Reference |
|---|---|---|---|---|
| S1A Peptidases | ||||
| Actinomadura viridilutea | DZ50 | KERDZ | KU550701 | [94] |
| Actinomadura keratinilytica | Cpt29 | KERAK-29 | ASU91959 | [95] |
| Streptomyces fradiae | Var. k11 | SFP2 | AJ784940 | [99] |
| Nocardiopsis sp. | TOA-1 | NAPase | AY151208 | [111] |
| S8A Peptidases | ||||
| Bacillus circulans | DZ100 | SAPDZ | AGN91700 | [100] |
| Bacillus licheniformis | RPk | KerRP | EU502844 | [96] |
| Stenotrophomonas maltophilia | BBE11-1 | KerSMD | KC814180 | [101] |
| Stenotrophomonas maltophilia | BBE11-1 | KerSMF | KC763971 | [101] |
| Bacillus pumilus | A1 | KerA1 | ACM47735 | [97] |
| Bacillus pumilus | CBS | SAPB | CAO03040 | [102] |
| Bacillus pumilus | KS12 | rK27 | HM219183 | [103] |
| Bacillus tequilensis | Q7 | KerQ7 | AKN20219 | [104] |
| Bacillus cereus | DCUW | Vpr | ACC94305 | [105,112] |
| Bacillus altitudinis | RBDV1 | KBALT | APZ77034 | [63] |
| Thermoactinomyces sp. | YT06 | YT06 Protease | WP_037995056 | [98] |
| Thermoactinomyces sp. | CDF | Protease C2 | ADD51544 | [106] |
| Meiothermus taiwanensis | WR-220 | rMtaKer | 5WSL | [107] |
| Brevibacillus sp. | WF146 | WF146 Protease | AAQ82911 | [108] |
| M4 Peptidases | ||||
| Geobacillus stearothermophilus | AD-11 | RecGEOker | KJ783444 | [109] |
| Pseudomonas aeruginosa | KS-1 | KerP | HM452163 | [110] |
| Protein | * pH | * Temp (°C) | Conditions | PT | CT |
|---|---|---|---|---|---|
| S1A Peptidases | |||||
| KERDZ | 11 | 80 | 10 g/L keratin azure, 50 mM bicarbonate-NaOH buffer, pH 11 mixed 1:1 with the enzyme, 30 min, 80 °C, 200 rpm (Abs595nm). | - | 2 mM CaCl2 |
| KERAK-29 | 10 | 70 | 1 mL of 10 g/L keratin azure, 100 mM Glycine-NaOH buffer mixed 1:1 with the enzyme, pH 10, 20 min, 70 °C (Abs595nm). | - | 5 mM MnSO4 |
| SFP2 | 10 | 60 | 5 mg keratin azure, 50 mM Tris-HCl, pH 8.5, 1 h, 37 °C (Abs595nm). | - | 10 mM DTT |
| NAPase | 12.5 | 60 | 60 mg wool keratin powder, Glycine-NaOH, pH 10 or 50 mM KCl-NaOH, pH 12.5, 30 °C, 2 h (Abs280nm). | Not specified | - |
| S8A Peptidases | |||||
| SAPDZ | 12.5 | 85 | 10 g/L keratin azure, 100 mM KClNaOH, 250 rpm, 20 min incubation, 85 °C (Abs595nm). | - | 5 mM CaCl2 |
| KerRP | 9 (11) | 60 (65–70) | 0.8% w/v keratin diluted 1:1 in enzyme, 1 h incubation, 60 °C (Abs280nm). | Not specified | - |
| KerSMD | 8 | 60 | 1% w/v soluble keratin, 50 mM Gly-NaOH, 20 min, 50 °C (Folin–Ciocalteu). | Not specified | - |
| KerSMF | 9 | 60 | 1% w/v soluble keratin, 50 mM Gly-NaOH, 20 min, 50 °C (Folin–Ciocalteu). | Not specified | - |
| KerA1 | 9 (10) | 60 (65) | 0.8% w/v keratin diluted 1:1 in enzyme solution, 1h, 50 °C (Abs280nm). | Not specified | - |
| SAPB | 10.6 | 65 | 1% keratin w/v, on 100 mM glycine-NaOH Buffer, pH 10.6, 30 min, 55 °C. 2 mM CaCl2 supplemented. | Not specified | - |
| rK27 | 9 | 70 | 20 mg feather powder, Gly-NaOH 50 mM, 1 h (Abs280nm). | Washed with Triton X-100 (1%), rinsed with water, autoclaved, dried in an oven at 60 °C for 1 h, milled then sieved with 2 mm pore size. | - |
| KerQ7 | 7 | 30 | 10 g/L keratin azure, 50 mM HEPES buffer, 30 min, 200 rpm (Abs595nm). | - | 1 mM CaCl2 |
| Vpr | 8.5 | 50 | 2% w/v chopped feather keratin, 50 °C, 15 min, pH 7.5. | - | - |
| KBALT | 8 | 85 | 5 mg keratin azure, 0.8 mL buffer, 15 min incubation, pH 6 to 12, 25 to 95 °C (Abs595nm). | - | - |
| YT06 protease | 8–9 | 65 | 1% soluble keratin, 50 mM Gly-NaOH, pH 9, 20 min (Folin–Ciocalteu). | Not specified | - |
| Protease C2 | 11 | 60–80 | 5% keratin powder, 50 mM Tris-HCl pH 8, 60 min 60 °C (Abs280nm). | 1000 °C incubation in DMSO for 2 h. Protein precipitated with acetone 2:1 v/v [133] | 0.5% β-ME |
| rMtaKer | 10 | 65 | 1% feather powder on 50 mM HEPES, pH8.0, 25–95 °C. Supplemented with 10 mM CaCl2, 150 mM NaCl (Ninhydrin). | Chicken feathers rinsed, air-dried, ground by ball mill. | - |
| WF146 protease | - | 80 | 10 mg of feathers, 50 °C or 80 °C, 1 ml Tris-HCl 50 mM buffer, pH 8.0, 10 mM CaCl2, multiple time points from 0 to 20 h (Abs280nm). | 70 Ethanol wash, rinse water, dry, cut 2–3 mm long | 1% β-ME |
| M4 Peptidases | |||||
| RecGEOker | 9 | 60 | 4 mg keratin azure, 50 mM Tris-HCl, pH 7.8, 1 h (Wool-Folin–Ciocalteu; Abs595nm). | - | - |
| KerP | 9 | 50 | 20 mg chicken feathers, Glycine-NaOH buffer, pH 10, 60 °C, 60 min (Abs280nm) | - | - |
| Protein | Metal ions (%) | Detergents (%) | Reducing Agents (%) | Solvents/Others (%) | ||
|---|---|---|---|---|---|---|
| S1A Peptidases | ||||||
| KERDZ | Ca2+ (270) Mg2+ (180) Fe2+ (145) | |||||
| KERAK-29 | Ca2+ (150) Mg2+ (110) Mn2+ (210) | Zwittergent (114) Tween-20 (130) Triton X-100 (132) Tween-80 (145) TTAB (116) CHAPS (140) | Sulfobetaine (135) LAS (118) SDS (115) CTAB (110) | β-ME (102) | H2O2 (170) | |
| SFP2 | Cu2+ (149) Ni2+ (116) | DTT (278) β-ME (235) | ||||
| NAPase | Isopropanol (130) | |||||
| S8A Peptidases | ||||||
| SAPDZ | Ca2+ (450) Mg2+ (195) Mn2+ (280) | Zn2+ (180) Cu2+ (110) Co2+ (113) | ||||
| KerRP | * Ca2+ | |||||
| KerSMD | Ca2+ (112) | Na2SO3 (116) | ||||
| KerSMF | ** Ca2+ (105) | Tween-20 (112) | Na2SO3 (115) DTT (115) | |||
| KerA1 | Ca2+ (123) Mg2+ (199) Na+ (135) | Tween 80 (113) | β-ME (Casein 100) (Keratin 192) | |||
| SAPB | Ca2+ (157) Mg2+ (112) Na+ (118) | LAS (114) Tween 80 (119) | Tween 20 (117) SDS (119) | β-ME (192) | Urea (165) H2O2 (168) | |
| rK27 | Stability only tested | Triton X-100 (677) Tween-80 (242) Saponin (461) Sodium Cholate (276) SDS (186) | DTT (267) β-ME (323) | NaClO (276) H2O2 (275) | ||
| KerQ7 | Ca2+ (417) Mg2+ (175) Mn2+ (250) | Ba2+ (121) Sn2+ (115) | ||||
| KBALT | Ca2+ (127) Mg2+ (134) | Zn2+ (129) Ba2+ (115) | SDS (128) | β-ME (102.5) | ||
| YT06 Protease | Mg2+ (118) Mn2+ (196) | Ni2+ (120) Ba2+ (115) | Tween-20 (170) | β-ME (623) | ||
| M4 Peptidases | ||||||
| RecGEOker | Mg2+ (112) Mn2+ (116) Zn2+ 1 mM (58); 10 mM (52) Ca2+ 1 mM (101); 10 mM (66) | Triton X-100 (115) Tween 40 (180) Tween 60 (133) | Tween 80 (122) Triton X-305 (153) | DTT (139) | ||
| Protein | Keratins (%) | Natural Proteins (%) | Modified Protein (%) | Esters and Others (%) | |||
|---|---|---|---|---|---|---|---|
| S1A Peptidases | |||||||
| KERDZ | Keratin (100) 2 | Gelatin (90) Casein (79) Albumin (75) Elastin (50) | Myoglobin (41) Hemoglobin (20) Collagen type 1/2 (0) | Azocasein (80) Azoalbumin (70) | BAEE (91) TAME (100) BCEE (95) BTEE (0) ATEE (0) | ||
| SFP2 | Keratin (100)2 | Casein (111) | |||||
| S8A Peptidases | |||||||
| SAPDZ | Keratin (100) | Gelatin (81) Casein (95) Albumin (72) | Hemoglobin (66) Collagen type 1/2 (0) | Azocasein (91) Keratin Azure (100) 1 | BAEE (0) BCEE (0) | BTEE (100) ATEE (95) | |
| KerSMD | Feather powder (54) Soluble keratin (1589) Wool (59) | Casein (2800) | Keratin Azure (100) 1 | ||||
| KerSMF | Feather powder (71) Soluble keratin (126) Wool (78) | Casein (92) | Keratin Azure (100) 1 | ||||
| KerA1 | Keratin (100) 2 | Gelatin (22) Casein (222) | Elastin (54) BSA (97) Egg albumin (4) | Azocasein (177) Azokeratin (92) | |||
| SAPB | Keratin (100)2 | Gelatin (146) Casein (153) | Bovine serum albumin (80) Egg albumin (18) Gluten (30) | Azocasein (123) Azokeratin (96) | BTEE (109) ATEE (115) | ||
| rK27 | Powdered chicken feather > haemoglobin > meat protein > hoof keratin > fibrin > elastin > gelatine > casein > BSA > azocasein > keratin azure | ||||||
| KerQ7 | Rabbit hair (88) Goat hair (74) Bovine hair (50) | Wool (12) Feather meal (116) Feather (100) 3 | |||||
| Vpr | Feather meal (50) Keratin (100) 2 | Gelatin (147) Casein (156) | Fibrin (145) Collagen (129) | ||||
| Protease C2 | Bovine hair (274) Feather (439) | Albumin (8571) Elastin (11) | Keratin azure (100) 1 Azocasein (102857) Azocoll (24000) | ||||
| M4 Peptidases | |||||||
| RecGEOker | Wool (100) 5 | Gelatin (92) Casein (95) | Albumin (37) Collagen type 1 (98) | ||||
| Trade Name | Source | EC Number | Substrate or Function | Supplier |
|---|---|---|---|---|
| Versazyme 1,3 | Bacillus licheniformis | 3.4.21.62/ S8 family | Improving nutritional value of poultry feed & prions degradation | Bioresource Int’l, Inc. |
| Ronozyme ProAct 2 | Nocardiopsis prasina | 3.4.21.-/serine protease | Improving nutritional value animal feed | DSM/Novozymes |
| Cibenza DP100 2 | Bacillus licheniformis PWD-1 | - | Improving nutritional value animal feed | Novus International |
| Pure Keratinase 100 3 | Bacillus licheniformis PWD-1 | - | Prion degradation from medical & dental instruments | Proteus Biotech |
| BioGuard Plus 3 | Proprietary blend of microorganisms – incl. keratinase producer | - | Cleaning drainpipes, septic tanks & digesters | RuShay Inc. |
| Keratoclean sensitive PB 3 | Bacillus licheniformis (PB333 keratinase) | - | Treatment acne, dead skin removal, promotes cell renewal | Proteus Biotech |
| Keratoclean Hydra PB 3 | Bacillus licheniformis | - | Removal of corns & call uses, acne, Hirsutism, peeling | Proteus Biotech |
| FixaFungus 3 | - | - | Treatment of toenail fungal infections | Proteus Biotech |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira Martinez, J.P.; Cai, G.; Nachtschatt, M.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Challenges and Opportunities in Identifying and Characterising Keratinases for Value-Added Peptide Production. Catalysts 2020, 10, 184. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020184
De Oliveira Martinez JP, Cai G, Nachtschatt M, Navone L, Zhang Z, Robins K, Speight R. Challenges and Opportunities in Identifying and Characterising Keratinases for Value-Added Peptide Production. Catalysts. 2020; 10(2):184. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020184
Chicago/Turabian StyleDe Oliveira Martinez, Juan Pinheiro, Guiqin Cai, Matthias Nachtschatt, Laura Navone, Zhanying Zhang, Karen Robins, and Robert Speight. 2020. "Challenges and Opportunities in Identifying and Characterising Keratinases for Value-Added Peptide Production" Catalysts 10, no. 2: 184. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020184





