Au-TiO2 Synthesized by a Microwave- and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst
Abstract
:1. Introduction
2. Results and Discussion
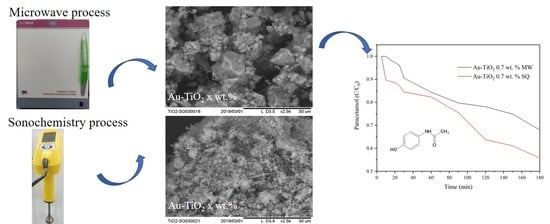
2.1. Structural Characterization of the Photocatalysts
2.2. Cyclic Voltammetric Response and Mott-Schottky Analysis
2.3. Photocatalytic Tests
3. Materials and Methods
3.1. Photocatalysts Synthesis
3.2. Physicochemical Characterization
3.3. Electrochemical Experiments
3.4. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranka, P.; Sethi, V.; Contractor, A.Q. One step electrodeposition of composite of PANI-PSS tubules with TiO2 nanoparticles and application as electronic sensor device. Sens. Actuators B Chem. 2018, 261, 11–21. [Google Scholar] [CrossRef]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Rosales, A.; Maury-Ramírez, A.; Gutiérrez, R.M.-D.; Guzmán, C.; Esquivel, K. SiO2@TiO2 Coating: Synthesis, Physical Characterization and Photocatalytic Evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Water Process Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Rasheed, T.; Adeel, M.; Nabeel, F.; Bilal, M.; Iqbal, H.M.N. TiO2/SiO2 decorated carbon nanostructured materials as a multifunctional platform for emerging pollutants removal. Sci. Total Environ. 2019, 688, 299–311. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Asadi, A.; Akbarzadeh, R.; Eslami, A.; Jen, T.-C.; Ozaveshe Oviroh, P. Effect of synthesis method on NS-TiO2 photocatalytic performance. Energy Procedia 2019, 158, 4542–4547. [Google Scholar] [CrossRef]
- Ingrosso, C.; Bianco, G.V.; Pifferi, V.; Guffanti, P.; Petronella, F.; Comparelli, R.; Agostiano, A.; Striccoli, M.; Palchetti, I.; Falciola, L.; et al. TiO2 Nanocrystals Decorated CVD Graphene Based Hybrid for UV-Light Active Photoanodes. Procedia Eng. 2016, 168, 396–402. [Google Scholar] [CrossRef]
- Astinchap, B.; Laelabadi, K.G. Effects of substrate temperature and precursor amount on optical properties and microstructure of CVD deposited amorphous TiO2 thin films. J. Phys. Chem. Solids 2019, 129, 217–226. [Google Scholar] [CrossRef]
- Chen, W.-F.; Mofarah, S.S.; Hanaor, D.A.H.; Koshy, P.; Chen, H.-K.; Jiang, Y.; Sorrell, C.C. Enhancement of Ce/Cr Codopant Solubility and Chemical Homogeneity in TiO2 Nanoparticles through Sol–Gel versus Pechini Syntheses. Inorg. Chem. 2018, 57, 7279–7289. [Google Scholar] [CrossRef] [PubMed]
- Solís-Casados, D.A.; Escobar-Alarcón, L.; Arrieta-Castañeda, A.; Haro-Poniatowski, E. Bismuth-Titanium Oxide nanopowders prepared by sol-gel method for photocatalytic applications. Mater. Chem. Phys. 2016, 172, 11–19. [Google Scholar] [CrossRef]
- Blanco-Vega, M.P.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Maya-Treviño, L.; Garza-Tovar, L.L.; Hernández-Ramírez, A.; Hinojosa-Reyes, L. Photocatalytic elimination of bisphenol A under visible light using Ni-doped TiO2 synthesized by microwave assisted sol-gel method. Mater. Sci. Semicond. Process. 2017, 71, 275–282. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Jafari, A.; Khademi, S.; Farahmandjou, M.; Darudi, A.; Rasuli, R. Structural and Optical Properties of Ce3+-Doped TiO2 Nanocrystals Prepared by Sol–Gel Precursors. J. Electron. Mater. 2018, 47, 6901–6908. [Google Scholar] [CrossRef]
- Tony, V.C.S.; Voon, C.H.; Lim, B.Y.; Al-Douri, Y.; Gopinath, S.C.B.; Arshad, M.K.M.; Ten, S.T.; Parmin, N.A.; Ruslinda, A.R. Synthesis of silicon carbide nanomaterials by microwave heating: Effect of types of carbon nanotubes. Solid State Sci. 2019, 98, 106023. [Google Scholar] [CrossRef]
- Kang, J.; Gao, L.; Zhang, M.; Pu, J.; He, L.; Ruan, R.; Omran, M.; Peng, J.; Chen, G. Synthesis of rutile TiO2 powder by microwave-enhanced roasting followed by hydrochloric acid leaching. Adv. Powder Technol. 2020, 31, 1140–1147. [Google Scholar] [CrossRef]
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2017, 13, 86–95. [Google Scholar] [CrossRef]
- Shetty, R.; Chavan, V.B.; Kulkarni, P.S.; Kulkarni, B.D.; Kamble, S.P. Photocatalytic Degradation of Pharmaceuticals Pollutants Using N-Doped TiO2 Photocatalyst: Identification of CFX Degradation Intermediates. Indian Chem. Eng. 2017, 59, 177–199. [Google Scholar] [CrossRef]
- Esquivel, K.; Nava, R.; Zamudio-Méndez, A.; González, M.V.; Jaime-Acuña, O.E.; Escobar-Alarcón, L.; Peralta-Hernández, J.M.; Pawelec, B.; Fierro, J.L.G. Microwave-assisted synthesis of (S)Fe/TiO2 systems: Effects of synthesis conditions and dopant concentration on photoactivity. Appl. Catal. B Environ. 2013, 140–141, 213–224. [Google Scholar] [CrossRef]
- Guo, W.; Lin, Z.; Wang, X.; Song, G. Sonochemical synthesis of nanocrystalline TiO2 by hydrolysis of titanium alkoxides. Microelectron. Eng. 2003, 66, 95–101. [Google Scholar] [CrossRef]
- Kaviyarasan, K.; Vinoth, V.; Sivasankar, T.; Asiri, A.M.; Wu, J.J.; Anandan, S. Photocatalytic and photoelectrocatalytic performance of sonochemically synthesized Cu2O@TiO2 heterojunction nanocomposites. Ultrason. Sonochem. 2019, 51, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Meskin, P.E.; Ivanov, V.K.; Barantchikov, A.E.; Churagulov, B.R.; Tretyakov, Y.D. Ultrasonically assisted hydrothermal synthesis of nanocrystalline ZrO2, TiO2, NiFe2O4 and Ni0.5Zn0.5Fe2O4 powders. Ultrason. Sonochem. 2006, 13, 47–53. [Google Scholar] [CrossRef]
- de Santiago Colín, D.M.; Martínez-Chávez, L.A.; Cuán, Á.; Elizalde-Peña, E.A.; Rivera, J.A.; Guzmán, C.; Escobar-Alarcón, L.; Esquivel, K. Sonochemical coupled synthesis of Cr-TiO2 supported on Fe3O4 structures and chemical simulation of the degradation mechanism of Malachite Green dye. J. Photochem. Photobiol. A Chem. 2018, 364, 250–261. [Google Scholar] [CrossRef]
- Zhu, L.; Chung, J.; Oh, W.-C. Rapid sonochemical synthesis of novel PbSe–graphene–TiO2 composite sonocatalysts with enhanced on decolorization performance and generation of ROS. Ultrason. Sonochem. 2015, 27, 252–261. [Google Scholar] [CrossRef]
- Shende, T.P.; Bhanvase, B.A.; Rathod, A.P.; Pinjari, D.V.; Sonawane, S.H. Sonochemical synthesis of Graphene-Ce-TiO2 and Graphene-Fe-TiO2 ternary hybrid photocatalyst nanocomposite and its application in degradation of crystal violet dye. Ultrason. Sonochem. 2018, 41, 582–589. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, Z.; Li, Y.; Yu, Z.; Liu, Q.; Li, J.; Feng, C.; Zhang, D. Sonochemical synthesis of TiO2 nanoparticles on graphene for use as photocatalyst. Ultrason. Sonochem. 2011, 18, 1082–1090. [Google Scholar] [CrossRef]
- Ambati, R.; Gogate, P.R. Ultrasound assisted synthesis of iron doped TiO2 catalyst. Ultrasonics Sonochem. 2018, 40, 91–100. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Hymavathi, B. X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson–Hall and size-strain plot methods. J. Asian Ceram. Soc. 2017, 5, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.; Hernández-Reséndiz, J.R.; Martínez-Chávez, A.; Velázquez-Castillo, R.; Escobar-Alarcón, L.; Esquivel, K. X-ray diffraction Rietveld structural analysis of Au–TiO2 powders synthesized by sol–gel route coupled to microwave and sonochemistry. J. Sol-Gel Sci. Technol. 2020, 95, 239–252. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, C. UV Raman Spectroscopic Studies on Titania: Phase Transformation and Significance of Surface Phase in Photocatalysis. In Environmentally Benign Photocatalysts; Anpo, M., Kamat, P.V., Eds.; Nanostructure Science and Technology; Springer: New York, NY, USA, 2010; pp. 153–184. ISBN 978-0-387-48441-9. [Google Scholar]
- Leyva-Porras, C.; Toxqui-Teran, A.; Vega-Becerra, O.; Miki-Yoshida, M.; Rojas-Villalobos, M.; García-Guaderrama, M.; Aguilar-Martínez, J.A. Low-temperature synthesis and characterization of anatase TiO2 nanoparticles by an acid assisted sol–gel method. J. Alloys Compd. 2015, 647, 627–636. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Lopez, T.; Cuevas, J.L.; Ilharco, L.; Ramírez, P.; Rodríguez-Reinoso, F.; Rodríguez-Castellón, E. XPS characterization and E. Coli DNA degradation using functionalized Cu/TiO2 nanobiocatalysts. Mol. Catal. 2018, 449, 62–71. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. One-pot synthesis of Au/N-TiO2 photocatalysts for environmental applications: Enhancement of dyes and NOx photodegradation. Powder Technol. 2019, 355, 793–807. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Nowaczyk, G.; Jancelewicz, M.; Pavlenko, M.; Załęski, K.; Jurga, S. Structural and XPS studies of PSi/TiO2 nanocomposites prepared by ALD and Ag-assisted chemical etching. Appl. Surf. Sci. 2015, 347, 777–783. [Google Scholar] [CrossRef]
- George, P.P.; Gedanken, A.; Perkas, N.; Zhong, Z. Selective oxidation of CO in the presence of air over gold-based catalysts Au/TiO2/C (sonochemistry) and Au/TiO2/C (microwave). Ultrason. Sonochem. 2008, 15, 539–547. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Arabatzis, I.M.; Stergiopoulos, T.; Andreeva, D.; Kitova, S.; Neophytides, S.G.; Falaras, P. Characterization and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Chenakin, S.; Kruse, N. Combining XPS and ToF-SIMS for assessing the CO oxidation activity of Au/TiO2 catalysts. J. Catal. 2018, 358, 224–236. [Google Scholar] [CrossRef]
- Feng, X.; Pan, F.; Zhao, H.; Deng, W.; Zhang, P.; Zhou, H.-C.; Li, Y. Atomic layer deposition enabled MgO surface coating on porous TiO2 for improved CO2 photoreduction. Appl. Catal. B Environ. 2018, 238, 274–283. [Google Scholar] [CrossRef]
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2/Au/TiO2 multilayer thin-film photoanodes synthesized by pulsed laser deposition for photoelectrochemical degradation of organic pollutants. Sep. Purif. Technol 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: the role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Zwijnenburg, A.; Goossens, A.; Sloof, W.G.; Crajé, M.W.J.; van der Kraan, A.M.; Jos de Jongh, L.; Makkee, M.; Moulijn, J.A. XPS and Mössbauer Characterization of Au/TiO2 Propene Epoxidation Catalysts. J. Phys. Chem. B 2002, 106, 9853–9862. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Xiao, L.; Chen, F. Preparation and characterization of TiO2 photocatalysts by Fe3+ doping together with Au deposition for the degradation of organic pollutants. Appl. Catal. B Environ. 2009, 88, 525–532. [Google Scholar] [CrossRef]
- Marken, F.; Bhambra, A.S.; Kim, D.-H.; Mortimer, R.J.; Stott, S.J. Electrochemical reactivity of TiO2 nanoparticles adsorbed onto boron-doped diamond surfaces. Electrochem. Commun. 2004, 6, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Bisquert, J. Cyclic Voltammetry Studies of Nanoporous Semiconductors. Capacitive and Reactive Properties of Nanocrystalline TiO2 Electrodes in Aqueous Electrolyte. J. Phys. Chem. B 2003, 107, 758–768. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Li, G.; Tang, C.; Sun, L. High-Quality Brookite TiO2 Flowers: Synthesis, Characterization, and Dielectric Performance. Cryst. Growth Des. 2009, 9, 3676–3682. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Hierarchical Nanotubular Anatase/Rutile/TiO2(B) Heterophase Junction with Oxygen Vacancies for Enhanced Photocatalytic H2 Production. Langmuir 2018, 34, 1883–1889. [Google Scholar] [CrossRef]
- Neppolian, B.; Wang, Q.; Jung, H.; Choi, H. Ultrasonic-assisted sol-gel method preparation of TiO2 nano-particles: Characterization, properties and 4-chlorophenol removal application. Ultrason. Sonochem. 2008, 15, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Olvera-Rodríguez, I.; Guzmán, C.; Medel, A.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. Microwave-assisted sol-gel synthesis of an Au-TiO2 photoanode for the advanced oxidation of paracetamol as model pharmaceutical pollutant. Electrochem. Commun. 2018, 96, 42–46. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Chironi, I.; Karatchevtseva, I.; Triani, G.; Sorrell, C.C. Single and mixed phase TiO2 powders prepared by excess hydrolysis of titanium alkoxide. Adv. Appl. Ceram. 2012, 111, 149–158. [Google Scholar] [CrossRef] [Green Version]














| Nominal Au Content (wt.%) | Ti (at.%) | O (at.%) | Au (at.%) | Au (wt.%) |
|---|---|---|---|---|
| 0 | --- | --- | --- | --- |
| 0.05 | 36.4 | 63.6 | --- | --- |
| 0.1 | 35.7 | 64.3 | --- | --- |
| 0.3 | 35.9 | 63.2 | 0.9 | 0.060 |
| 0.7 | 33.0 | 66.0 | 1.0 | 0.069 |
| 1 | 36.1 | 62.8 | 1.1 | 0.073 |
| Nominal Au Content (wt.%) | Ti (at.%) | O (at.%) | Au (at.%) | Au (wt.%) |
|---|---|---|---|---|
| 0 | --- | --- | --- | --- |
| 0.05 | 36.3 | 63.7 | ---- | ---- |
| 0.1 | 36.3 | 63.1 | 0.6 | 0.041 |
| 0.3 | 35.9 | 63.2 | 0.8 | 0.054 |
| 0.7 | 33.4 | 65.7 | 0.9 | 0.062 |
| 1 | 35.1 | 63.9 | 1.0 | 0.068 |
| Material | (Efb) a Anatase | (Nd) b Anatase | (Efb) a Brookite | (Nd) b Brookite |
|---|---|---|---|---|
| TiO2 (SQ) | −0.437 | 2.1 × 1027 | −0.880 | 2.5 × 1027 |
| Au-TiO2 0.05 wt.% (SQ) | −0.581 | 6.9 × 1027 | −1.065 | 8.7 × 1027 |
| Au-TiO2 0.1 wt.% (SQ) | −0.991 | 1.0 × 1028 | −0.533 | 6.0 × 1027 |
| Au-TiO2 0.3 wt.% (SQ) | −0.921 | 2.0 × 1027 | −0.782 | 3.6 × 1027 |
| Au-TiO2 0.7 wt.% (SQ) | −1.004 | 3.3 × 1027 | −0.787 | 4.2 × 1027 |
| Au-TiO2 1.0 wt.% (SQ) | −0.814 | 1.7 × 1027 | −0.445 | 1.9 × 1027 |
| TiO2 (MW) | −0.980 | 1.9 × 1028 | −0.421 | 1.1 × 1028 |
| Au-TiO2 0.05 wt.% (MW) | −0.964 | 1.6 × 1028 | −0.404 | 8.2 × 1028 |
| Au-TiO2 0.1 wt.% (MW) | −0.954 | 1.6 × 1028 | −0.400 | 7.8 × 1028 |
| Au-TiO2 0.3 wt.% (MW) | −1.019 | 6.6 × 1028 | −0.384 | 2.6 × 1028 |
| Au-TiO2 0.7 wt.% (MW) | −1.082 | 8.9 × 1028 | −0.309 | 1.2 × 1028 |
| Au-TiO2 1.0 wt.% (MW) | −0.910 | 1.3 × 1028 | -0.321 | 2.2 × 1027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, R.; Hernández-Reséndiz, J.R.; Cruz-Ramírez, M.; Velázquez-Castillo, R.; Escobar-Alarcón, L.; Ortiz-Frade, L.; Esquivel, K. Au-TiO2 Synthesized by a Microwave- and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst. Catalysts 2020, 10, 1052. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091052
Hernández R, Hernández-Reséndiz JR, Cruz-Ramírez M, Velázquez-Castillo R, Escobar-Alarcón L, Ortiz-Frade L, Esquivel K. Au-TiO2 Synthesized by a Microwave- and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst. Catalysts. 2020; 10(9):1052. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091052
Chicago/Turabian StyleHernández, Rafael, José Rosendo Hernández-Reséndiz, Marisela Cruz-Ramírez, Rodrigo Velázquez-Castillo, Luis Escobar-Alarcón, Luis Ortiz-Frade, and Karen Esquivel. 2020. "Au-TiO2 Synthesized by a Microwave- and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst" Catalysts 10, no. 9: 1052. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091052