Biocatalysis at Extreme Temperatures: Enantioselective Synthesis of both Enantiomers of Mandelic Acid by Transesterification Catalyzed by a Thermophilic Lipase in Ionic Liquids at 120 °C
Abstract
:1. Introduction
2. Results
2.1. Ethanolysis of (R, S)-1 in Isooctane at Different Temperatures
2.2. Ethanolysis of (R, S)-1 in RTILs at Different Temperatures
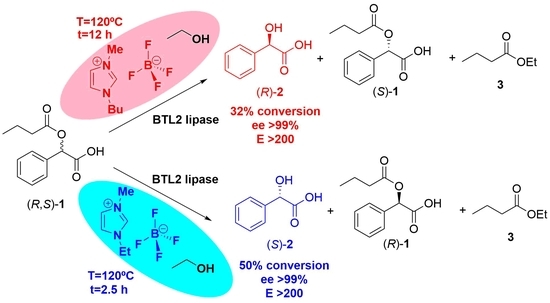
2.2.1. Ethanolysis of (R, S)-1 Using RTILs Based on 1-Butyl-3-methyl Imidazolium (BMIM) as Solvent
2.2.2. Ethanolysis of (R, S)-1 Using RTILs Based on 1-Ethyl-3-methyl Imidazolium (EMIM) as Solvent
3. Discussion
4. Materials
4.1. Synthesis of (R,S) 2-(Butyryloxy)-2-phenylacetic Acid
4.2. Resolution of 2-(Butyryloxy)-2-phenylacetic Acid by Alcoholysis Reaction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiltschi, B.; Cernava, T.; Dennig, A.; Casas, M.G.; Geier, M.; Gruber, S.; Haberbauer, M.; Heidinger, P.; Acero, E.H.; Kratzer, R.; et al. Enzymes revolutionize the bioproduction of value-added compounds: From enzyme discovery to special applications. Biotechnol. Adv. 2020, 40, 51. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. New frontiers in biocatalysis for sustainable synthesis. Curr. Opin. Green Sustain. Chem. 2020, 21, 22–26. [Google Scholar] [CrossRef]
- Huang, X.; Cao, M.; Zhao, H. Integrating biocatalysis with chemocatalysis for selective transformations. Curr. Opin. Chem. Biol. 2020, 55, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Amatuni, A.; Renata, H. Recent advances in the chemoenzymatic synthesis of bioactive natural products. Curr. Opin. Chem. Biol. 2020, 55, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.A.; Brady, D.; Bode, M.L.L. The Hitchhiker’s guide to biocatalysis: Recent advances in the use of enzymes in organic synthesis. Chem. Sci. 2020, 11, 2587–2605. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, B.A.; Hyster, T.K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- de Gonzalo, G.; Domínguez de María, P. Biocatalysis: An Industrial Perspective; Royal Society of Chemistry: London, UK, 2018. [Google Scholar] [CrossRef]
- Woodley, J.M. Accelerating the implementation of biocatalysis in industry. Appl. Microbiol. Biotechnol. 2019, 103, 4733–4739. [Google Scholar] [CrossRef]
- Hughes, G.; Lewis, J.C. Introduction: Biocatalysis in Industry. Chem. Rev. 2018, 118, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Domínguez de María, P.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as useful tool in asymmetric synthesis: An assessment of recently granted patents (2014–2019). Catalysts 2019, 9, 802. [Google Scholar] [CrossRef] [Green Version]
- Alcántara, A.R. Biotransformations in Drug Synthesis: A Green and Powerful Tool for Medicinal Chemistry. J. Med. Chem.Drug. Des. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Hoyos, P.; Pace, V.; Alcántara, A.R. Chiral Building Blocks for Drugs Synthesis via Biotransformations. In Asymmetric Synthesis of Drugs and Natural Products; Nag, A., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 346–448. [Google Scholar]
- Alcántara, A.R. Biocatalysis and Pharmaceuticals: A Smart Tool for Sustainable Development. Catalysts 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Truppo, M.D. Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, K.; Lutz, S. Recent developments and challenges of biocatalytic processes in the pharmaceutical industry. Curr. Opin. Green Sustain. Chem. 2018, 11, 58–64. [Google Scholar] [CrossRef]
- Lalor, F.; Fitzpatrick, J.; Sage, C.; Byrne, E. Sustainability in the biopharmaceutical industry: Seeking a holistic perspective. Biotechnol. Adv. 2019, 37, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D. Broadening the Scope of Biocatalysis in Sustainable Organic Synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Kumar, S.; Dangi, A.K.; Shukla, P.; Baishya, D.; Khare, S.K. Thermozymes: Adaptive strategies and tools for their biotechnological applications. Bioresour. Technol. 2019, 278, 372–382. [Google Scholar] [CrossRef]
- Han, H.W.; Ling, Z.M.; Khan, A.; Virk, A.K.; Kulshrestha, S.; Li, X.K. Improvements of thermophilic enzymes: From genetic modifications to applications. Bioresour. Technol. 2019, 279, 350–361. [Google Scholar] [CrossRef]
- González-Siso, M.-I. Editorial for the Special Issue: Thermophiles and Thermozymes. Microorganisms 2019, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Atalah, J.; Caceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hait, S.; Mallik, S.; Basu, S.; Kundu, S. Finding the generalized molecular principles of protein thermal stability. Proteins 2020, 88, 788–808. [Google Scholar] [CrossRef] [PubMed]
- Liszka, M.J.; Clark, M.E.; Schneider, E.; Clark, D.S. Nature Versus Nurture: Developing Enzymes That Function Under Extreme Conditions. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Ed.; Annual Reviews: Palo Alto, CA, USA, 2012; Volume 3, pp. 77–102. [Google Scholar]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Dumorne, K.; Cordova, D.C.; Astorga-Elo, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microb. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef] [PubMed]
- Daiha, K.D.; Angeli, R.; de Oliveira, S.D.; Almeida, R.V. Are lipases still important biocatalysts? A study of scientific publications and patents for technological forecasting. PLoS ONE 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of lipase-catalyzed reactions for organic synthesis: A recent update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.Q.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; Housaindokht, M.R.; Monhemi, H. Molecular mechanism of enzyme tolerance against organic solvents: Insights from molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 122, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Elleuche, S.; Schroder, C.; Antranikian, G. Lipolytic extremozymes from psychro- and (hyper-)thermophilic prokaryotes and their potential for industrial applications. In Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, P.H., Ed.; Springer International Publishing Ag: Cham, Switzerland, 2016; Volume 1, pp. 351–374. [Google Scholar]
- Lajis, A.F.B. Realm of Thermoalkaline Lipases in Bioprocess Commodities. J. Lipids 2018, 2018, 5659683. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Lopez, C.; Godoy, C.; de las Rivas, B.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R.; Martinez-Ripoll, M.; Hermoso, J.A. Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehata, M.; Timucin, E.; Venturini, A.; Sezerman, O.U. Understanding thermal and organic solvent stability of thermoalkalophilic lipases: Insights from computational predictions and experiments. J. Mol. Model. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp nov and Geobacillus uzenensis sp nov from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Dannert, C.; Sztajer, H.; Stocklein, W.; Menge, U.; Schmid, R.D. Screening, purification and properties of a thermophilic lipase from Bacillus thermocatenulatus. Biochim. Biophys. Acta Lipids Lipid Metab. 1994, 1214, 43–53. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C.; Rua, M.L.; Schmid, R.D. Bacillus thermocatenulatus lipase: A thermoalkalophilic lipase with interesting properties. Biochem. Soc. Trans. 1997, 25, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Dannert, C.; Rua, M.L.; Atomi, H.; Schmid, R.D. Thermoalkalophilic lipase of Bacillus thermocatenulatus. 1. Molecular cloning, nucleotide sequence, purification and some properties. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1301, 105–114. [Google Scholar] [CrossRef]
- Carrasco-Lopez, C.; Godoy, C.; de las Rivas, B.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R.; Martinez-Ripoll, M.; Hermoso, J.A. Crystallization and preliminary X-ray diffraction studies of the BTL2 lipase from the extremophilic microorganism Bacillus thermocatenulatus. Acta Crystallogr. F Struct. Biol. Commun. 2008, 64, 1043–1045. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.M.F.; Somers, N.A.; Kazlauskas, R.J.; Brush, T.S.; Zocher, F.; Enzelberger, M.M.; Bornscheuer, U.T.; Horsman, G.P.; Mezzetti, A.; Schmidt-Dannert, C.; et al. Mapping the substrate selectivity of new hydrolases using colorimetric screening: Lipases from Bacillus thermocatenulatus and Ophiostoma piliferum, esterases from Pseudomonas fluorescens and Streptomyces diastatochromogenes. Tetrahedron Asymmetry 2001, 12, 545–556. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fernandez-Lorente, G.; Rua, M.L.; Guisan, J.M.; Fernandez-Lafuente, R. Evaluation of the lipase from Bacillus thermocatenulatus as an enantioselective biocatalyst. Tetrahedron Asymmetry 2003, 14, 3679–3687. [Google Scholar] [CrossRef]
- Palomo, J.M.; Ortiz, C.; Fuentes, M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Use of immobilized lipases for lipase purification via specific lipase-lipase interactions. J. Chromatogr. A 2004, 1038, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Segura, R.L.; Fernandez-Lorente, G.; Pernas, M.; Rua, M.L.; Guisan, J.M.; Fernandez-Lafuente, R. Purification, immobilization, and stabilization of a lipase from Bacillus thermocatenulatus by interfacial adsorption on hydrophobic supports. Biotechnol. Prog. 2004, 20, 630–635. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process. Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Godoy, C.A.; Mendes, A.A.; Lopez-Gallego, F.; Grazu, V.; de las Rivas, B.; Palomo, J.M.; Hermoso, J.; Fernandez-Lafuente, R.; Guisan, J.M. Solid-phase chemical amination of a lipase from Bacillus thermocatenulatus to improve its stabilization via covalent immobilization on highly activated glyoxyl-agarose. Biomacromolecules 2008, 9, 2553–2561. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mateo, C.; Godoy, C.; Pessela, B.C.C.; Rodrigues, D.S.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Guisan, J.M. The co-operative effect of physical and covalent protein adsorption on heterofunctional supports. Process. Biochem. 2009, 44, 757–763. [Google Scholar] [CrossRef]
- Godoy, C.A.; de las Rivas, B.; Filice, M.; Fernandez-Lorente, G.; Guisan, J.M.; Palomo, J.M. Enhanced activity of an immobilized lipase promoted by site-directed chemical modification with polymers. Process. Biochem. 2010, 45, 534–541. [Google Scholar] [CrossRef]
- Godoy, C.A.; de las Rivas, B.; Bezbradica, D.; Bolivar, J.M.; Lopez-Gallego, F.; Fernandez-Lorente, G.; Guisan, J.M. Reactivation of a thermostable lipase by solid phase unfolding/refolding Effect of cysteine residues on refolding efficiency. Enzyme Microb. Technol. 2011, 49, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; de las Rivas, B.; Grazu, V.; Montes, T.; Manuel Guisan, J.; Lopez-Gallego, F. Glyoxyl-Disulfide Agarose: A Tailor-Made Support for Site-Directed Rigidification of Proteins. Biomacromolecules 2011, 12, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; Fernandez-Lorente, G.; de las Rivas, B.; Filice, M.; Guisan, J.M.; Palomo, J.M. Medium engineering on modified Geobacillus thermocatenulatus lipase to prepare highly active catalysts. J. Mol. Catal. B Enzym. 2011, 70, 144–148. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Abian, O.; Manuel Guisan, J. Altering the Interfacial Activation Mechanism of a Lipase by Solid-Phase Selective Chemical Modification. Biochemistry 2012, 51, 7028–7036. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; Romero, O.; de la Rivas, B.; Mateo, C.; Fernandez-Lorente, G.; Guisan, J.M.; Palomo, J.M. Changes on enantioselectivity of a genetically modified thermophilic lipase by site-directed oriented immobilization. J. Mol. Catal. B Enzym. 2013, 87, 121–127. [Google Scholar] [CrossRef]
- Marciello, M.; Bolivar, J.M.; Filice, M.; Mateo, C.; Guisan, J.M. Preparation of Lipase-Coated, Stabilized, Hydrophobic Magnetic Particles for Reversible Conjugation of Biomacromolecules. Biomacromolecules 2013, 14, 602–607. [Google Scholar] [CrossRef]
- Bautista-Barrufet, A.; Lopez-Gallego, F.; Rojas-Cervellera, V.; Rovira, C.; Pericas, M.A.; Guisan, J.M.; Gorostiza, P. Optical Control of Enzyme Enantioselectivity in Solid Phase. ACS Catal. 2014, 4, 1004–1009. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; Velez, A.M.; Giordano, R.C.; Giordano, R.d.L.C.; de Castro, H.F. Evaluation of immobilized lipases on poly-hydroxybutyrate beads to catalyze biodiesel synthesis. Int. J. Biol. Macromol. 2012, 50, 503–511. [Google Scholar] [CrossRef]
- Herranz, S.; Marciello, M.; Olea, D.; Hernandez, M.; Domingo, C.; Velez, M.; Gheber, L.A.; Guisan, J.M.; Moreno-Bondi, M.C. Dextran-Lipase Conjugates as Tools for Low Molecular Weight Ligand Immobilization in Microarray Development. Anal. Chem. 2013, 85, 7060–7068. [Google Scholar] [CrossRef] [Green Version]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalysed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Godoy, C.A. New Strategy for the Immobilization of Lipases on Glyoxyl-Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation. Int. J. Mol. Sci. 2017, 18, 2130. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Tejedor, D.; de las Rivas, B.; Palomo, J.M. Ultra-Small Pd(0) Nanoparticles into a Designed Semisynthetic Lipase: An Efficient and Recyclable Heterogeneous Biohybrid Catalyst for the Heck Reaction under Mild Conditions. Molecules 2018, 23, 2358. [Google Scholar] [CrossRef] [Green Version]
- Romero, O.; de las Rivas, B.; Lopez-Tejedor, D.; Palomo, J.M. Effect of Site-Specific Peptide-Tag Labeling on the Biocatalytic Properties of Thermoalkalophilic Lipase from Geobacillus thermocatenulatus. ChemBioChem 2018, 19, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perez, S.; Fernandez-Lorente, G.; Romero, O.; Guisan, J.M.; Lopez-Gallego, F. Fabrication of heterogeneous biocatalyst tethering artificial prosthetic groups to obtain omega-3-fatty acids by selective hydrolysis of fish oils. RSC Adv. 2016, 6, 97659–97663. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Godoy, C.A.; de las Rivas, B.; Guisan, J.M. Site-directing an intense multipoint covalent attachment (MCA) of mutants of the Geobacillus thermocatenulatus lipase 2 (BTL2): Genetic and chemical amination plus immobilization on a tailor-made support. Process. Biochem. 2014, 49, 1324–1331. [Google Scholar] [CrossRef]
- Kajiwara, S.; Yamada, R.; Matsumoto, T.; Ogino, H. N-linked glycosylation of thermostable lipase from Bacillus thermocatenulatus to improve organic solvent stability. Enzyme Microb. Technol. 2020, 132. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; Klett, J.; Di Geronimo, B.; Hermoso, J.A.; Guisan, J.M.; Carrasco-Lopez, C. Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2. Int. J. Mol. Sci. 2019, 20, 5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, E.; Karkhane, A.A.; Yakhchali, B.; Shamsara, M.; Aminzadeh, S.; Torktaz, I.; Hosseini, M.; Safari, Z. Study of the effect of F17A mutation on characteristics of Bacillus thermocatenulatus lipase expressed in Pichia pastoris using in silico and experimental methods. Biotech. Appl. Biochem. 2014, 61, 264–273. [Google Scholar] [CrossRef]
- Goodarzi, N.; Karkhane, A.A.; Mirlohi, A.; Tabandeh, F.; Torktas, I.; Aminzadeh, S.; Yakhchali, B.; Shamsara, M.; Ghafouri, M.A.-S. Protein engineering of Bacillus thermocatenulatus lipase via deletion of the alpha 5 helix. Appl. Biochem. Biotech. 2014, 174, 339–351. [Google Scholar] [CrossRef]
- Yenenler, A.; Venturini, A.; Burduroglu, H.C.; Sezerman, O.U. Investigating the structural properties of the active conformation BTL2 of a lipase from Geobacillus thermocatenulatus in toluene using molecular dynamic simulations and engineering BTL2 via in-silico mutation. J. Mol. Model. 2018, 24, 13. [Google Scholar] [CrossRef]
- Yukselen, O.; Timucin, E.; Sezerman, U. Predicting the impact of mutations on the specific activity of Bacillus thermocatenulatus lipase using a combined approach of docking and molecular dynamics. J. Mol. Recognit. 2016, 29, 466–475. [Google Scholar] [CrossRef]
- Khaleghinejad, S.H.; Motalleb, G.; Karkhane, A.A.; Aminzadeh, S.; Yakhchali, B. Study the effect of F17S mutation on the chimeric Bacillus thermocatenulatus lipase. J. Genet. Eng. Biotechnol. 2016, 14, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.H.; Meng, X.H.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme stability and activity in non-aqueous reaction systems: A mini review. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic liquids as a potential solvent for lipase-catalysed reactions: A review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Moniruzzaman, M.; Goto, M. Recent advances of enzymatic reactions in ionic liquids: Part II. Biochem. Eng. J. 2020, 154, 23. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- de los Rios, A.P.; Irabien, A.; Hollmann, F.; Fernandez, F.J.H. Ionic Liquids: Green Solvents for Chemical Processing. J. Chem. 2013, 2013, 402172. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Terreni, M.; Pagani, G.; Ubiali, D.; Fernandez-Lafuente, R.; Mateo, C.; Guisan, J.M. Modulation of penicillin acylase properties via immobilization techniques: One-pot chemoenzymatic synthesis of cephamandole from cephalosporin C. Bioorg. Med. Chem. Lett. 2001, 11, 2429–2432. [Google Scholar] [CrossRef]
- Furlenmeier, A.; Quitt, P.; Vogler, K.; Lanz, P. 6-Acyl Derivatives of Aminopenicillanic Acid. U.S. Patent US3957758A, 18 May 1976. [Google Scholar]
- Su, X.P.; Bhongle, N.N.; Pflum, D.; Butler, H.; Wald, S.A.; Bakale, R.P.; Senanayake, C.H. A large-scale asymmetric synthesis of (S)-cyclohexylphenyl glycolic acid. Tetrahedron Asymmetry 2003, 14, 3593–3600. [Google Scholar] [CrossRef]
- Glushkov, R.G.; Ovcharova, I.M.; Muratov, M.A.; Kaminka, M.E.; Mashkovsky, M.D. Synthesis and pharmacological activity of new oxyaminoalkylxanthines and dialkylaminoalkylxanthines 1,4-diazepino and pyrazino[1,2,3-g,h] purine derivatives. Khimiko-Farmatsevticheskii Zhurnal 1977, 11, 30–35. [Google Scholar]
- Bousquet, A.; Musolino, A. Hydroxyacetic Ester Derivatives, Namely (R)-methyl 2-(sulfonyloxy)-2-(chlorophenyl)acetates, Preparation Method, and Use as Synthesis Intermediates for Clopidogrel. WO9918110A1, 15 April 1999. [Google Scholar]
- Bast, A.; Leurs, R.; Timmerman, H. Cyclandelate as a calcium modulating agent in rat cerebral-cortex. Drugs 1987, 33, 67–74. [Google Scholar] [CrossRef]
- Gokce, M.; Utku, S.; Gur, S.; Ozkul, A.; Gumus, F. Synthesis, in vitro cytotoxic and antiviral activity of cis-[Pt(R(-) and S(+)-2-alpha,-hydroxybenzylbenzimidazole)(2)Cl-2] complexes. Eur. J. Med. Chem. 2005, 40, 135–141. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fujimatsu, I.; Komatsu, K. Purification and characterization of the nitrilase from Alcaligenes faecalis ATCC-8750 responsible for enantioselective hydrolysis of mandelonitrile. J. Ferment. Bioeng. 1992, 73, 425–430. [Google Scholar] [CrossRef]
- Yamamoto, K.; Oishi, K.; Fujimatsu, I.; Komatsu, K.I. Production of R-(-)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC-8750. Appl. Environ. Microbiol. 1991, 57, 3028–3032. [Google Scholar] [CrossRef] [Green Version]
- Poterala, M.; Dranka, M.; Borowiecki, P. Chemoenzymatic Preparation of Enantiomerically Enriched (R)-(-)-Mandelic Acid Derivatives: Application in the Synthesis of the Active Agent Pemoline. Eur. J. Org. Chem. 2017, 2017, 2290–2304. [Google Scholar] [CrossRef]
- Arroyo, M.; de la Mata, I.; Garcia, J.L.; Barredo, J.L. Biocatalysis for Industrial Production of Active Pharmaceutical Ingredients (APIs); Academic Press Ltd.: Cambridge, MA, USA; Elsevier Science Ltd.: London, UK, 2017; pp. 451–473. [Google Scholar] [CrossRef]
- Martin, J.R.; Nus, M.; Gago, J.V.S.; Sanchez-Montero, J.M. Selective esterification of phthalic acids in two ionic liquids at high temperatures using a thermostable lipase of Bacillus thermocatenulatus: A comparative study. J. Mol. Catal. B Enzym. 2008, 52–53, 162–167. [Google Scholar] [CrossRef]
- Pizzilli, A.; Zoppi, R.; Hoyos, P.; Gómez, S.; Gatti, F.G.; Hernáiz, M.J.; Alcántara, A.R. First stereoselective acylation of a primary diol possessing a prochiral quaternary center mediated by lipase TL from Pseudomonas stutzeri. Tetrahedron 2015, 71, 9172–9176. [Google Scholar] [CrossRef] [Green Version]
- Chamorro, S.; Sanchez-Montero, J.M.; Alcantara, A.R.; Sinisterra, J.V. Treatment of Candida rugosa lipase with short-chain polar organic solvents enhances its hydrolytic and synthetic activities. Biotechnol. Lett. 1998, 20, 499–505. [Google Scholar] [CrossRef]
- Borreguero, I.; Carvalho, C.M.L.; Cabral, J.M.S.; Sinisterra, J.V.; Alcantara, A.R. Enantioselective properties of Fusarium solani pisi cutinase on transesterification of acyclic diols: Activity and stability evaluation. J. Mol. Catal. B Enzym. 2001, 11, 613–622. [Google Scholar] [CrossRef]
- Chamorro, S.; Alcántara, A.R.; de la Casa, R.M.; Sinisterra, J.V.; Sánchez-Montero, J.M. Small water amounts increase the catalytic behaviour of polar organic solvents pre-treated Candida rugosa lipase. J. Mol. Catal. B Enzym. 2001, 11, 939–947. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Martínez-Alzamora, F.; Moreno, S.P.; Valero, F.; Rúa, M.L.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Heptyl oleate synthesis as useful tool to discriminate between lipases, proteases and other hydrolases in crude preparations. Enzyme Microb, Technol. 2002, 31, 283–288. [Google Scholar] [CrossRef]
- Alcántara, A.R.; De María, P.D.; Fernández, M.; Hernaíz, M.J.; Sánchez-Montero, J.M.; Sinisterra, J.V. Resolution of racemic acids, esters and amines by Candida rugosa lipase in slightly hydrated organic media. Food Technol. Biotechnol. 2004, 42, 343–354. [Google Scholar]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Bardsley, W.G. SIMFIT—A Computer Package for Simulation, Curve-Fitting and Statistical-Analysis using Life-Science Models; Plenum Press Div. Plenum Publishing Corp.: New York, NY, USA, 1993; pp. 455–458. [Google Scholar]
- Zheng, D.X.; Dong, L.; Huang, W.J.; Wu, X.H.; Nie, N. A review of imidazolium ionic liquids research and development towards working pair of absorption cycle. Renew. Sust. Energ. Rev. 2014, 37, 47–68. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Domanska, U.; Marciniak, A. Solubility of ionic liquid [emim] [PF6] in alcohols. J. Phys. Chem. B 2004, 108, 2376–2382. [Google Scholar] [CrossRef]
- Heintz, A. Recent developments in thermodynamics and thermophysics of non-aqueous mixtures containing ionic liquids. A review. J. Chem. Thermodyn. 2005, 37, 525–535. [Google Scholar] [CrossRef]
- Sahandzhieva, K.; Tuma, D.; Breyer, S.; Kamps, A.P.S.; Maurer, G. Liquid-liquid equilibrium in mixtures of the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate and an alkanol. J. Chem. Eng. Data 2006, 51, 1516–1525. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodriguez, A. Study on the phase behaviour and thermodynamic properties of ionic liquids containing imidazolium cation with ethanol at several temperatures. J. Chem. Thermodyn. 2007, 39, 978–989. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Tekin, A.; Safarov, J.; Shahverdiyev, A.; Hassel, E. Densities and excess, apparent, and partial molar volumes of binary mixtures of BMIMBF4 plus ethanol as a function of temperature, pressure, and concentration. Int. J. Thermophys. 2008, 29, 505–533. [Google Scholar] [CrossRef]
- Domanska, U. Solubilities and thermophysical properties of ionic liquids. Pure Appl. Chem. 2005, 77, 543–557. [Google Scholar] [CrossRef]
- Guo, Y.M.; Wang, X.; Tao, X.Y.; Shen, W.G. Liquid-liquid equilibrium and heat capacity measurements of the binary solution {ethanol+1-butyl-3-methylimidazolium hexafluorophosphate}. J. Chem. Thermodyn. 2017, 115, 342–351. [Google Scholar] [CrossRef]
- Varela, L.M.; Mendez-Morales, T.; Carrete, J.; Gomez-Gonzalez, V.; Docampo-Alvarez, B.; Gallego, L.J.; Cabeza, O.; Russina, O. Solvation of molecular cosolvents and inorganic salts in ionic liquids: A review of molecular dynamics simulations. J. Mol. Liq. 2015, 210, 178–188. [Google Scholar] [CrossRef]
- Secundo, F.; Carrea, G.; Tarabiono, C.; Gatti-Lafranconi, P.; Brocca, S.; Lotti, M.; Jaeger, K.E.; Puls, M.; Eggert, T. The lid is a structural and functional determinant of lipase activity and selectivity. J. Mol. Catal. B Enzym. 2006, 39, 166–170. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Kazlauskas, R.J.; Weissfloch, A.N.E.; Rappaport, A.T.; Cuccia, L.A. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalysed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991, 56, 2656–2665. [Google Scholar] [CrossRef]
- Borreguero, I.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Rumbero, A.; Hermoso, J.A.; Alcántara, A.R. Regioselective resolution of 1,n-diols catalysed by lipases: A rational explanation of the enzymatic selectivity. J. Mol. Catal. B Enzym. 2001, 11, 1013–1024. [Google Scholar] [CrossRef]
- Borreguero, I.; Sinisterra, J.V.; Rumbero, A.; Hermoso, J.A.; Martínez-Ripoll, M.; Alcántara, A.R. Acyclic phenylalkanediols as substrates for the study of enzyme recognition. Regioselective acylation by porcine pancreatic lipase: A structural hypothesis for the enzymatic selectivity. Tetrahedron 1999, 55, 14961–14974. [Google Scholar] [CrossRef]
- Filice, M.; Romero, O.; Abian, O.; de las Rivas, B.; Palomo, J.M. Low ionic liquid concentration in water: A green and simple approach to improve activity and selectivity of lipases. RSC Adv. 2014, 4, 49115–49122. [Google Scholar] [CrossRef] [Green Version]
- Maraite, A.; Hoyos, P.; Carballeira, J.D.; Cabrera, Á.C.; Ansorge-Schumacher, M.B.; Alcántara, A.R. Lipase from Pseudomonas stutzeri: Purification, homology modelling and rational explanation of the substrate binding mode. J. Mol. Catal. B Enzym. 2013, 87, 88–98. [Google Scholar] [CrossRef]
- Min, B.; Park, J.; Sim, Y.K.; Jung, S.; Kim, S.H.; Song, J.K.; Kim, B.T.; Park, S.Y.; Yun, J.; Park, S.; et al. Hydrogen-bonding-driven enantioselective resolution against the Kazlauskas rule to afford gamma-amino alcohols by Candida rugosa lipase. ChemBioChem 2015, 16, 77–82. [Google Scholar] [CrossRef]
- Nascimento, P.A.M.; Pereira, J.F.B.; Santos-Ebinuma, V.D. Insights into the effect of imidazolium-based ionic liquids on chemical structure and hydrolytic activity of microbial lipase. Bioprocess. Biosyst. Eng. 2019, 42, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Modulation of immobilized lipase enantioselectivity via chemical amination. Adv. Synth. Catal. 2007, 349, 1119–1127. [Google Scholar] [CrossRef]







| Medium | T (°C) | VS 1 | VR 1 | VS/VR | tMAX 4 | [C] max 5 | P 6 | [(S)-2] 7 | [(R)-2] 7 | E7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | isooctane | 40 | 0.015 2 0.027 3 | 0.008 2 0.014 3 | 1.9 2 1.9 3 | 0 | 0 | nd | |||
| #2 | isooctane | 70 | 0.083 2 | 0.063 2 0.332 3 | 1.32 2 | 24 | 2.26 | 0.09 | 1.44 | 0 | >200 |
| #3 | isooctane | 90 | 0.110 2 | 0.108 2 | 1.022 | 4.0 | 3.75 | nd |
| RTIL | T (°C) | VS 1 | VR 1 | VMAX/Vmin 2 | tMAX 3 | [C] max 4 | P 5 | [(S)-2] 6 | [(R)-2] 6 | E 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #4 | BMIM BF4 | 90 | 3.85 | 0.76 | 5.1 | 10 | 13.6 | 1.36 | 15.42 | 13.6 | 1.1 |
| #5 | BMIM BF4 | 120 | - | 8.14 | - | 36 | 18.8 | 0.52 | 0 | 18.8 | >200 |
| #6 | BMIM-PF6 | 90 | 2.0 | 0.31 | 6.4 | 24 | 17.0 | 0.71 | 14.0 | 0 | >200 |
| #7 | BMIM-PF6 | 120 | 0.04 | 0.96 | 24 | 8 | 6.3 | 0.79 | 0 | 3.06 | >200 |
| #8 | EMIM-BF4 | 90 | 4.08 | 0.52 | 7.8 | 12 | 18.2 | 3.03 | 18.2 | 0 | >200 |
| #9 | EMIM-BF4 | 120 | 53.4 | 0 | - | 2.5 | 29.2 | 11.7 | 28.1 | 0 | >200 |
| #10 | EMIM-PF6 | 90 | 1.86 | - | - | 240 | 16.2 | 0.81 | 2.28 | 0.06 | 51.3 |
| #11 | EMIM-PF6 | 120 | 1.3 | - | - | 54 | 9.8 | 0.18 | 8.52 | 0 | >200 |
| RTIL | MW 1 (g/mol) | Density 1 (g/mL) | [RTIL], M | [EtOH], M | Molar Fraction XRTIL |
|---|---|---|---|---|---|
| BMIMBF4 | 226.02 | 1.21 | 4.28 | 3.42 | 0.56 |
| BMIMPF6 | 284.19 | 1.38 | 3.89 | 3.42 | 0.53 |
| EMIMBF4 | 197.97 | 1.29 | 5.22 | 3.42 | 0.60 |
| EMIMPF6 | 256.13 | 1.48 | 4.62 | 3.42 | 0.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Martín, J.; Khiari, O.; Alcántara, A.R.; Sánchez-Montero, J.M. Biocatalysis at Extreme Temperatures: Enantioselective Synthesis of both Enantiomers of Mandelic Acid by Transesterification Catalyzed by a Thermophilic Lipase in Ionic Liquids at 120 °C. Catalysts 2020, 10, 1055. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091055
Ramos-Martín J, Khiari O, Alcántara AR, Sánchez-Montero JM. Biocatalysis at Extreme Temperatures: Enantioselective Synthesis of both Enantiomers of Mandelic Acid by Transesterification Catalyzed by a Thermophilic Lipase in Ionic Liquids at 120 °C. Catalysts. 2020; 10(9):1055. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091055
Chicago/Turabian StyleRamos-Martín, Jesús, Oussama Khiari, Andrés R. Alcántara, and Jose María Sánchez-Montero. 2020. "Biocatalysis at Extreme Temperatures: Enantioselective Synthesis of both Enantiomers of Mandelic Acid by Transesterification Catalyzed by a Thermophilic Lipase in Ionic Liquids at 120 °C" Catalysts 10, no. 9: 1055. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091055