Efficient Photocatalytic Degradation of Gaseous Benzene and Toluene over Novel Hybrid PIL@TiO2/m-GO Composites
Abstract
:1. Introduction
2. Analyzing the Data and Results
2.1. Characterization
2.1.1. XRD Patterns
2.1.2. FESEM and TEM Images
2.1.3. X-Ray Photoelectron Spectroscopy (XPS)
2.1.4. Spectroscopy Results for FTIR
2.1.5. Raman Spectra
2.1.6. Band Gap Determination and UV–Vis Spectra
2.2. Photocatalytic Activity towards Degradation of Benzene and Toluene
2.2.1. Evaluating the Photocatalytic Results
2.2.2. Kinetic Studies of Benzene and Toluene Photocatalytic Degradation
- (CA0): Initial pollutants concentrations,
- (CA): Final pollutants concentrations,
- (K’): Rate constant of the first-order reaction, and
- (t): Accommodation time.
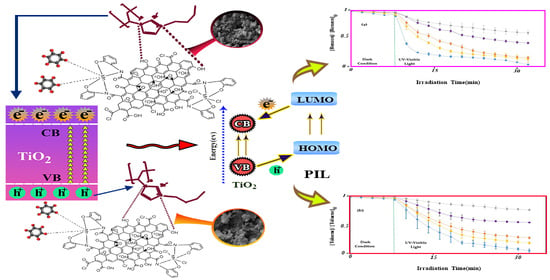
2.2.3. Photocatalytic Benzene and Toluene Degradation Mechanism
2.3. Photocatalytic Stability
2.3.1. XRD of PIL(low)@TiO2@m-GO after the Photocatalytic Reaction
2.3.2. FESEM and TEM of PIL(low)@TiO2@m-GO after the Photocatalytic Reaction
2.3.3. Recycle Ability of PIL(low)/TiO2/m-GO
3. Experimental
3.1. Materials
3.2. Preparation and Synthesis
3.2.1. Preparation of Graphene Oxide (GO)
3.2.2. Preparation of Modified Graphene Oxide (m-GO)
3.2.3. Preparation of the Pure TiO2
3.2.4. Preparation of 3-Butyl-1-Vinylimidazolium Bromide Monomer
3.2.5. Preparation of Poly 3-Butyl-1-Vinylimidazolium Bromide
3.2.6. Preparation of PIL@TiO2
3.3. Instrumentation
3.4. Photocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, J.; Kakosimos, K.; Jensen, S.S.; Hertel, O.; Sørensen, M.; Gulliver, J.; Ketzel, M. The spatial relationship between traffic-related air pollution and noise in two Danish cities: Implications for health-related studies. Sci. Total Environ. 2020, 726, 138577. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Hao, J.; Wang, X.; Wang, S.; Chai, F.; Li, M. Air pollution and control action in Beijing. J. Clean. Prod. 2016, 112, 1519–1527. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, A.-I.; Lee, J.-C.; Saianand, G.; Lee, K.-P.; Sonar, P.; Dharmarajan, R.; Hou, Y.; Ann, K.-Y.; Kannan, V.; Kim, W.-J. Recent progress in the abatement of hazardous pollutants using photocatalytic TiO2-based building materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Li, G.; An, T.; Fu, J. The health risk attenuation by simultaneous elimination of atmospheric VOCs and POPs from an e-waste dismantling workshop by an integrated de-dusting with decontamination technique. Chem. Eng. J. 2016, 301, 299–305. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de Los Ríos, A.P.; Quesada-Medina, J. Ionic liquid technology to recover volatile organic compounds (VOCs). J. Hazard. Mater. 2017, 321, 484–499. [Google Scholar] [CrossRef]
- Bennett, J.W.; Inamdar, A.A. Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins 2015, 7, 3785. [Google Scholar] [CrossRef] [Green Version]
- McMichael, A.J. Carcinogenicity of benzene, toluene and xylene: Epidemiological and experimental evidence. IARC Sci. Publ. 1988, 85, 3. [Google Scholar]
- Weon, S.; Kim, J.; Choi, W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound. Appl. Catal. B Environ. 2018, 220, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Matsushima, S.; Hojo, H.; Einaga, H. Photocatalytic oxidation process for treatment of gas phase benzene using Ti3+ self-doped TiO2 microsphere with sea urchin-like structure. Chem. Eng. J. 2020, 402, 126220. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.; Lu, J.; Xiao, J.; Lei, Y.; Yu, Z. BiVO4/α-Fe2O3 catalytic degradation of gaseous benzene: Preparation, characterization and photocatalytic properties. Appl. Surf. Sci. 2018, 427, 141–147. [Google Scholar] [CrossRef]
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286. [Google Scholar] [CrossRef]
- Da Costa Filho, B.M.; Silva, G.V.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Ozonation and ozone-enhanced photocatalysis for VOC removal from air streams: Process optimization, synergy and mechanism assessment. Sci. Total Environ. 2019, 687, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.-C.; Park, Y.-K. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Chen, P.; Cui, W.; Wang, H.; Li, J.; Sun, Y.; Zhou, Y.; Zhang, Y.; Dong, F. The importance of intermediates ring-opening in preventing photocatalyst deactivation during toluene decomposition. Appl. Catal. B Environ. 2020, 272, 118977. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, Y.; Xia, X.; Liu, X.; Li, H. Synthesis of Mo-doped TiO2 nanowires/reduced graphene oxide composites with enhanced photodegradation performance under visible light irradiation. RSC Adv. 2016, 6, 23809–23815. [Google Scholar] [CrossRef]
- Xiao, W.-Z.; Xu, L.; Rong, Q.-Y.; Dai, X.-Y.; Cheng, C.-P.; Wang, L.-L. Two-dimensional H-TiO2/MoS2 (WS2) van der Waals heterostructures for visible-light photocatalysis and energy conversion. Appl. Surf. Sci. 2020, 504, 144425. [Google Scholar] [CrossRef]
- Dolat, D.; Quici, N.; Kusiak-Nejman, E.; Morawski, A.W.; Puma, G.L. One-step, hydrothermal synthesis of nitrogen, carbon co-doped titanium dioxide (N, CTiO2) photocatalysts. Effect of alcohol degree and chain length as carbon dopant precursors on photocatalytic activity and catalyst deactivation. Appl. Catal. B Environ. 2012, 115, 81–89. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Wang, Y.; Zheng, N.; Yang, L.; Yu, H.; Yu, Y. A novel Ag-BiOBr-rGO photocatalyst for enhanced ketoprofen degradation: Kinetics and mechanisms. Sci. Total Environ. 2019, 678, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Ge, S.; Li, H.; Su, Y.; Li, Q.; Zhou, W.; Gao, B.; Yue, Q. Synchronous synthesis of Cu2O/Cu/rGO@carbon nanomaterials photocatalysts via the sodium alginate hydrogel template method for visible light photocatalytic degradation. Sci. Total Environ. 2019, 693, 133657. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Li, M.; He, S.; Ma, Q.; Wang, X. Construction of Z-scheme and pn heterostructure: Three-dimensional porous g-C3N4/graphene oxide-Ag/AgBr composite for high-efficient hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118384. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Şen, B.; Akdere, E.H.; Şavk, A.; Gültekin, E.; Paralı, Ö.; Göksu, H.; Şen, F. A novel thiocarbamide functionalized graphene oxide supported bimetallic monodisperse Rh-Pt nanoparticles (RhPt/TC@GO NPs) for Knoevenagel condensation of aryl aldehydes together with malononitrile. Appl. Catal. B Environ. 2018, 225, 148–153. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Chen, S.-S.; Feng, J.-J.; Lin, X.-X.; Wang, W.; Wang, A.-J. Dicationic ionic liquid mediated fabrication of Au@Pt nanoparticles supported on reduced graphene oxide with highly catalytic activity for oxygen reduction and hydrogen evolution. Appl. Surf. Sci. 2018, 441, 438–447. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl. Catal. B Environ. 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, Z.; Cui, Y.; Xie, G.; Jin, Y.; Guo, L.; Xu, Y.; Zhang, Q.; Li, X. Catalytic performance of ionic liquid for dehydrochlorination reaction: Excellent activity and unparalled stability. Appl. Catal. B Environ. 2019, 255, 117757. [Google Scholar] [CrossRef]
- Qi, K.; Qi, H.; Yang, J.; Wang, G.-C.; Selvaraj, R.; Zheng, W. Experimental and theoretical DFT+D investigations regarding to various morphology of cuprous oxide nanoparticles: Growth mechanism of ionic liquid-assisted synthesis and photocatalytic activities. Chem. Eng. J. 2017, 324, 347–357. [Google Scholar] [CrossRef]
- Wang, D.; Xu, L.; Nai, J.; Bai, X.; Sun, T. Morphology-controllable synthesis of nanocarbons and their application in advanced symmetric supercapacitor in ionic liquid electrolyte. Appl. Surf. Sci. 2019, 473, 1014–1023. [Google Scholar] [CrossRef]
- Gai, H.; Zhong, C.; Liu, X.; Qiao, L.; Zhang, X.; Xiao, M.; Song, H. Poly (ionic liquid)-supported gold and ruthenium nanoparticles toward the catalytic wet air oxidation of ammonia to nitrogen under mild conditions. Appl. Catal. B Environ. 2019, 258, 117972. [Google Scholar] [CrossRef]
- Huang, T.; Long, M.-C.; Wang, X.-L.; Wu, G.; Wang, Y.-Z. One-step preparation of poly (ionic liquid)-based flexible electrolytes by in-situ polymerization for dendrite-free lithium ion batteries. Chem. Eng. J. 2019, 375, 122062. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Highly selective poly(ethylene oxide)/ionic liquid electrolyte membranes containing CrO3 for CO2/N2 separation. Chem. Eng. J. 2019, 356, 312–317. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Hou, W.; Shan, W.; Li, J.; Zhou, Y.; Wang, J. Mesoporous poly (ionic liquid) supported palladium (II) catalyst for oxidative coupling of benzene under atmospheric oxygen. Appl. Surf. Sci. 2018, 427, 575–583. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Gao, Q.; Li, J.; Shen, Y.; Zhu, X. An aspirated in-syringe device fixed with ionic liquid and β-cyclodextrin-functionalized CNTs/TiO2 for rapid adsorption and visible-light-induced photocatalytic activity. New J. Chem. 2019, 43, 9345–9353. [Google Scholar] [CrossRef]
- Fausey, C.L.; Zucker, I.; Shaulsky, E.; Zimmerman, J.B.; Elimelech, M. Removal of arsenic with reduced graphene oxide-TiO2-enabled nanofibrous mats. Chem. Eng. J. 2019, 375, 122040. [Google Scholar] [CrossRef]
- Wang, M.; Hua, J.; Yang, Y. Fabrication of CDs/CdS-TiO2 ternary nano-composites for photocatalytic degradation of benzene and toluene under visible light irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 102–109. [Google Scholar] [CrossRef]
- Lang, X.; Saianand, G.; Fu, W.; Ramakrishna, S. Photocatalytic water splitting utilizing electrospun semiconductors for solar hydrogen generation: Fabrication, Modification and Performance. Bull. Chem. Soc. Jpn. 2021, 94, 8–20. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Ceylan, H.; Ozgit-Akgun, C.; Erkal, T.S.; Donmez, I.; Garifullin, R.; Tekinay, A.B.; Usta, H.; Biyikli, N.; Guler, M.O. Size-controlled conformal nanofabrication of biotemplated three-dimensional TiO2 and ZnO nanonetworks. Sci. Rep. 2013, 3, 2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, J.; Wang, M.; Li, Z.; Liu, H.; He, P.; Yang, X.; Li, J. Preparation and properties of nanostructure anatase TiO2 monoliths using 1-butyl-3-methylimidazolium tetrafluoroborate room-temperature ionic liquids as template solvents. Cryst. Growth Des. 2005, 5, 1643–1649. [Google Scholar] [CrossRef]
- Chen, H.; Nambu, A.; Wen, W.; Graciani, J.; Zhong, Z.; Hanson, J.C.; Fujita, E.; Rodriguez, J.A. Reaction of NH3 with titania: N-doping of the oxide and TiN formation. J. Phys. Chem. C 2007, 111, 1366–1372. [Google Scholar] [CrossRef]
- Saleem, H.; Habib, A. Study of band gap reduction of TiO2 thin films with variation in GO contents and use of TiO2/Graphene composite in hybrid solar cell. J. Alloys Compd. 2016, 679, 177–183. [Google Scholar] [CrossRef]
- Freitas, R.G.; Santanna, M.A.; Pereira, E.C. Preparation and characterization of TiO2 nanotube arrays in ionic liquid for water splitting. Electrochim. Acta 2014, 136, 404–411. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Chen, X.; Pan, G.; Huo, Y. Ionic-liquid-assisted growth of flower-like TiO2 film on Ti substrate with high photocatalytic activity. J. Mol. Catal. A Chem. 2013, 373, 12–17. [Google Scholar] [CrossRef]
- Jana, S.; Trivedi, M.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R.K. Characterization of physical, thermal and spectral properties of biofield treated O-aminophenol. Pharm. Anal. Acta 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Song, L.; Yu, B.; Yang, W.; Wang, B.; Hu, Y.; Yuen, R.K.K. One-pot surface functionalization and reduction of graphene oxide with long-chain molecules: Preparation and its enhancement on the thermal and mechanical properties of polyurea. Chem. Eng. J. 2014, 236, 233–241. [Google Scholar] [CrossRef]
- Zhao, W.; Kido, G.; Hara, K.; Noguchi, H. Characterization of neutralized graphite oxide and its use in electric double layer capacitors. J. Electroanal. Chem. 2014, 712, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Ajdari, F.B.; Kowsari, E.; Ehsani, A. Ternary nanocomposites of conductive polymer/functionalized GO/MOFs: Synthesis, characterization and electrochemical performance as effective electrode materials in pseudocapacitors. J. Solid State Chem. 2018, 265, 155–166. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectros. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, R.K. A facile method of synthesizing ammonia modified graphene oxide for efficient removal of uranyl ions from aqueous medium. RSC Adv. 2015, 5, 77192–77203. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Nadri, H.R.; Maghsoodi, M.; Ehsani, A.; Mahmoudi, H.; Eshkalak, S.K.; Chinnappan, A.; Jayathilak, W.; Ramakrishna, S. Electrochemical performance of Silsesquioxane-GO loaded with alkoxy substituted ammonium-based ionic liquid and POAP for supercapacitor. Electrochim. Acta 2020, 354, 136663. [Google Scholar] [CrossRef]
- Ashtiani, A.A.; Kowsari, E.; Haddadi-Asl, V.; Yousefi, M.; Naderi, H.R.; Chinnappan, A.; Ramakrishna, S. Pseudocapacitive efficiency of covalently Cr-complex with L-histidine-methyl ester as a ligand graphene oxide blended with conducting polymer (POAP) as electrode material in supercapacitor. J. Mol. Liq. 2020, 315, 113697. [Google Scholar] [CrossRef]
- Eshlaghi, M.A.; Kowsari, E.; Ehsani, A.; Akbari-Adergani, B.; Hekmati, M. Functionalized graphene oxide GO-[imi-(CH2)2-NH2] as a high efficient material for electrochemical sensing of lead: Synthesis surface and electrochemical characterization. J. Electroanal. Chem. 2020, 858, 113784. [Google Scholar] [CrossRef]
- Takehira, H.; Karim, M.R.; Shudo, Y.; Fukuda, M.; Mashimo, T.; Hayami, S. Modulating the Work Function of Graphene by Pulsed Plasma Aided Controlled Chlorination. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Kowsari, E.; Morad, F.; Seifvand, N.; Bazri, B.; Karimi, M. Synthesis of reduced graphene oxide functionalized with methyl red dye and its role in enhancing photoactivity in TiO2–IL/WO3 composite for toluene degradation. Res. Chem. Intermed. 2020, 46, 1217–1234. [Google Scholar] [CrossRef]
- Wei, H.; Ma, Y.; Luo, J.; Wu, K.-H.; Xie, W.; Wen, G.; Chiang, C.-L.; Yan, W.; Perathoner, S.; Centi, G. Creation of N-C=O active groups on N-doped CNT as an efficient CarboCatalyst for solvent-free aerobic coupling of benzylamine. Carbon 2020, 170, 338–346. [Google Scholar] [CrossRef]
- Sivaranjini, B.; Mangaiyarkarasi, R.; Ganesh, V.; Umadevi, S. Vertical alignment of liquid crystals over a functionalized flexible substrate. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhang, K.; Chan, H.S.O.; Wu, J. Nanostructured MnO2/graphene composites for supercapacitor electrodes: The effect of morphology, crystallinity and composition. J. Mater. Chem. 2012, 22, 1845–1851. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOx Nanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- Ehsani, A.; Kowsari, E.; Boorboor Ajdari, F.; Safari, R.; Mohammad Shiri, H. Influence of newly synthesized geminal dicationic ionic liquid on electrochemical and pseudocapacitance performance of conductive polymer electroactive film. J. Colloid Interface Sci. 2017, 505, 1158–1164. [Google Scholar] [CrossRef]
- Ehsani, A.; Kowsari, E.; Boorboor Ajdari, F.; Safari, R.; Mohammad Shiri, H. Sulfonated graphene oxide and its nanocomposites with electroactive conjugated polymer as effective pseudocapacitor electrode materials. J. Colloid Interface Sci. 2017, 497, 258–265. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Ehsani, A.; Schorowski, M.; Ameri, T. New synthesized ionic liquid functionalized graphene oxide: Synthesis, characterization and its nanocomposite with conjugated polymer as effective electrode materials in an energy storage device. Electrochim. Acta 2018, 292, 789–804. [Google Scholar] [CrossRef]
- Vahur, S.; Kriiska, A.; Leito, I. Investigation of the adhesive residue on the flint insert and the adhesive lump found from the Pulli Early Mesolithic settlement site (Estonia) by micro-ATR-FT-IR spectroscopy. Est. J. Archaeol. 2011, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; Suzuki, M.; El-Moneim, A.A. Facile synthesis of MnO2/graphene electrode by two-steps electrodeposition for energy storage application. Int. J. Electrochem. Sci. 2014, 9, 8340–8354. [Google Scholar]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. 2014, 4, 5044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerapandian, M.; Seo, Y.-T.; Yun, K.; Lee, M.-H. Graphene oxide functionalized with silver@silica–polyethylene glycol hybrid nanoparticles for direct electrochemical detection of quercetin. Biosens. Bioelectron. 2014, 58, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, W.W.; Irmer, G. Hydration and speciation studies of Mn2+ in aqueous solution with simple monovalent anions (ClO4−, NO3−, Cl−, Br−). Dalt. Trans. 2013, 42, 14460–14472. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ma, F.; Li, T.; Li, G. Solvothermal synthesis of N-doped TiO2 nanoparticles using different nitrogen sources, and their photocatalytic activity for degradation of benzene. Chin. J. Catal. 2013, 34, 2263–2270. [Google Scholar] [CrossRef]
- Seifvand, N.; Kowsari, E. TiO2/in-situ reduced GO/functionalized with an IL-Cr complex as a ternary photocatalyst composite for efficient carbon monoxide deterioration from air. Appl. Catal. B Environ. 2017, 206, 184–193. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Ao, C.H.; Lee, S.C.; Hou, M.F. Enhanced photocatalytic degradation of VOCs using Ln3+–TiO2 catalysts for indoor air purification. Chemosphere 2005, 59, 787–800. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, X.; Hou, Y.; Chen, X.; Wu, L.; Fu, X. Degradation of benzene over a zinc germanate photocatalyst under ambient conditions. Environ. Sci. Technol. 2008, 42, 7387–7391. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Deng, J.; Zhang, K.; Hou, Z.; Zhao, X.; Zhang, X.; Zhang, K.; Wei, R.; Dai, H. Coupled Palladium–Tungsten Bimetallic Nanosheets/TiO2 Hybrids with Enhanced Catalytic Activity and Stability for the Oxidative Removal of Benzene. Environ. Sci. Technol. 2019, 53, 5926–5935. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, J.; Tao, L.; Gong, M.; Zhimin, L.; Chen, Y. Photocatalytic degradation of gaseous benzene over TiO2/Sr2CeO4: Kinetic model and degradation mechanisms. J. Hazard. Mater. 2007, 139, 323–331. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Xu, Y.; Lu, J.; Huang, L.; Yang, Z. Photocatalytic degradation of gaseous benzene with H3PW12O40/TiO2/palygorskite composite catalyst. J. Saudi Chem. Soc. 2017, 21, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Huang, H.; Xie, R.; Feng, Q.; Fang, R.; Shu, Y.; Zhan, Y.; Ye, X.; Zhong, C. Enhanced degradation of gaseous benzene by a Fenton reaction. RSC Adv. 2016, 7, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Kask, M.; Bolobajev, J.; Krichevskaya, M. Gas-phase photocatalytic degradation of acetone and toluene, and their mixture in the presence of ozone in continuous multi-section reactor as possible air post-treatment for exhaust from pulsed corona discharge. Chem. Eng. J. 2020, 399, 125815. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Domen, K.; Arakawa, H. A new type of water splitting system composed of two different TiO2 photocatalysts (anatase, rutile) and a IO3−/I− shuttle redox mediator. Chem. Phys. Lett. 2001, 344, 339–344. [Google Scholar] [CrossRef]
- Abe, A.; Yamashita, K. Theoretical study on the photo-stimulated desorption of Xe from an oxidized Si (0 0 1) surface. Chem. Phys. Lett. 2001, 343, 143–150. [Google Scholar] [CrossRef]
- Ghasemi, S.; Setayesh, S.R.; Habibi-Yangjeh, A.; Hormozi-Nezhad, M.R.; Gholami, M.R. Assembly of CeO2–TiO2 nanoparticles prepared in room temperature ionic liquid on graphene nanosheets for photocatalytic degradation of pollutants. J. Hazard. Mater. 2012, 199–200, 170–178. [Google Scholar] [CrossRef]
- Chávez, A.M.; Solís, R.R.; Beltrán, F.J. Magnetic graphene TiO2-based photocatalyst for the removal of pollutants of emerging concern in water by simulated sunlight aided photocatalytic ozonation. Appl. Catal. B Environ. 2020, 262, 118275. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.-K. Enhanced photocatalytic decomposition of VOCs by visible-driven photocatalyst combined Cu-TiO2 and activated carbon fiber. Process Saf. Environ. Prot. 2018, 119, 164–171. [Google Scholar] [CrossRef]
- Shoushtarian, F.; Moghaddam, M.R.A.; Kowsari, E. Efficient regeneration/reuse of graphene oxide as a nanoadsorbent for removing basic Red 46 from aqueous solutions. J. Mol. Liq. 2020, 312, 113386. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Karami, A. Synthesis of TiO2 nano powder by the sol-gel method and its use as a photocatalyst. J. Iran. Chem. Soc. 2010, 7, S154–S160. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.; Ran, T.; Gao, H.; Liao, Y. Preparation and application of poly (zwitterionic ionic liquid) to enhance the photocatalytic activity of TiO2. J. Mater. Sci. 2016, 51, 7186–7198. [Google Scholar] [CrossRef]
- Meinke, W.W.; Taylor, J.K. Analytical Chemistry: Key to Progress on National Problems: Proceedings; National Bureau of Standards: Washington, DC, USA, 1972; Volume 13.
- Suryanarayana, C.; Grant, N.M. X-Ray Diffraction—A Practical Approach; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Lee, J.-C.; Gopalan, A.-I.; Saianand, G.; Lee, K.-P.; Kim, W.-J. Manganese and Graphene Included Titanium Dioxide Composite Nanowires: Fabrication, Characterization and Enhanced Photocatalytic Activities. Nanomaterials 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of visible light photocatalytic graphene embedded rutile titanium (IV) oxide composite nanowires and enhanced NOx removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]















| Photocatalyst | Band Gap (ev) |
|---|---|
| TiO2 | 3.2 |
| PIL(high)/TiO2 | 2.53 |
| PIL(low)/TiO2 | 2.15 |
| PIL(low)/TiO2/m-GO | 2.1 |
| Photocatalyst | Benzene | Toluene |
|---|---|---|
| μmol. g−1. h−1 | μmol. g−1. h−1 | |
| TiO2 | 0.7 | 1.9 |
| PIL(high)/TiO2 | 0.98 | 3.64 |
| PIL(low)/TiO2 | 1.43 | 5.85 |
| PIL(low)/TiO2/GO | 1.52 | 6.59 |
| PIL(low)/TiO2/m-GO | 1.62 | 7.67 |
| Photocatalyst | Benzene Degradation Kinetic Data | |||
|---|---|---|---|---|
| First-order data | Second-order data | |||
| K’ (min−1) | R2 | K″ [(ppmv)−1.min−1] | R2 | |
| TiO2 | 0/0279 | 0/9427 | 0/0007 | 0/9162 |
| PIL(high)/TiO2 | 0/0425 | 0/972 | 0/0013 | 0/9932 |
| PIL(low)/TiO2 | 0/0891 | 0/8415 | 0/0046 | 0/9518 |
| PIL(low)/TiO2/GO | 0/1108 | 0/9333 | 0/0072 | 0/9476 |
| PIL(low)/TiO2/m-GO | 0/136 | 0/6361 | 0/0133 | 0/5465 |
| Photocatalyst | Toluene Degradation Kinetic Data | |||
| First-order data | Second-order data | |||
| K’ (min−1) | R2 | K″ [(ppmv)−1.min−1] | R2 | |
| TiO2 | 0.013 | 0/9093 | 0/0003 | 0/9427 |
| PIL(high)/TiO2 | 0/0328 | 0/7225 | 0/0009 | 0/8242 |
| PIL(low)/TiO2 | 0/0659 | 0/8963 | 0/0025 | 0/9896 |
| PIL(low)/TiO2/GO | 0/0879 | 0/8858 | 0/0043 | 0/9875 |
| PIL(low)/TiO2/m-GO | 0/1425 | 0/9705 | 0/0153 | 0/7053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shajari, S.; Kowsari, E.; Seifvand, N.; Boorboor Ajdari, F.; Chinnappan, A.; Ramakrishna, S.; Saianand, G.; Dashti Najafi, M.; Haddadi-Asl, V.; Abdpour, S. Efficient Photocatalytic Degradation of Gaseous Benzene and Toluene over Novel Hybrid PIL@TiO2/m-GO Composites. Catalysts 2021, 11, 126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010126
Shajari S, Kowsari E, Seifvand N, Boorboor Ajdari F, Chinnappan A, Ramakrishna S, Saianand G, Dashti Najafi M, Haddadi-Asl V, Abdpour S. Efficient Photocatalytic Degradation of Gaseous Benzene and Toluene over Novel Hybrid PIL@TiO2/m-GO Composites. Catalysts. 2021; 11(1):126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010126
Chicago/Turabian StyleShajari, Shayeste, Elaheh Kowsari, Naemeh Seifvand, Farshad Boorboor Ajdari, Amutha Chinnappan, Seeram Ramakrishna, Gopalan Saianand, Mohammad Dashti Najafi, Vahid Haddadi-Asl, and Soheil Abdpour. 2021. "Efficient Photocatalytic Degradation of Gaseous Benzene and Toluene over Novel Hybrid PIL@TiO2/m-GO Composites" Catalysts 11, no. 1: 126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010126