A Comprehensive Study of Coke Deposits on a Pt-Sn/SBA-16 Catalyst during the Dehydrogenation of Propane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Atomic Absorption Spectroscopy (AAS) and N2 Adsorption-Desorption
2.2. Catalytic Tests
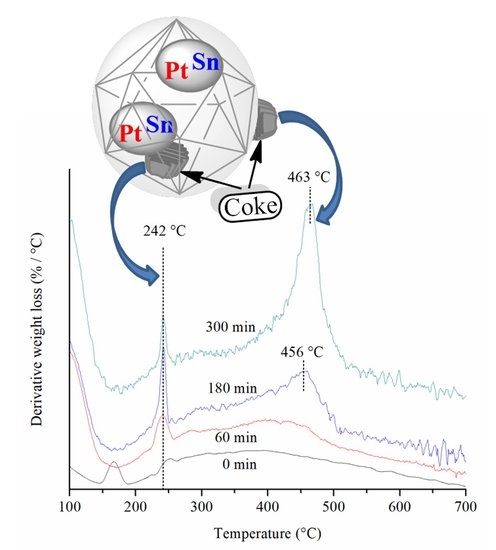
2.3. Thermogravimetric Analysis (TGA)
2.4. Raman Analysis
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Transmission Electron Microscopy (TEM)
3. Materials and Methods
3.1. Atomic Absorption Spectroscopy (AAS)
3.2. N2 Adsorption-Desorption
3.3. Thermogravimetric Analysis (TGA)
3.4. Raman Spectroscopy
3.5. X-ray Photoelectron Spectroscopy (XPS)
3.6. Transmission Electron Microscopy (TEM)
3.7. Support and Catalyst Synthesis
3.7.1. Supports
3.7.2. Catalysts
3.8. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Wu, Z.; Zhang, G.; Sheng, H.; Tian, J.; Duan, Z.; Sohn, H.; Kropf, A.J.; Wu, T.; Krause, T.R.; et al. Promotion of Pd nanoparticles by Fe and formation of a Pd3Fe intermetallic alloy for propane dehydrogenation. Catal. Today 2019, 323, 123–128. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, J.; Rong, J.; Liu, C.; Dai, Z.; Bao, J.; Da, Z.; Zheng, H. Defect-driven unique stability of Pt/carbon nanotubes for propane dehydrogenation. Appl. Surf. Sci. 2019, 464, 146–152. [Google Scholar] [CrossRef]
- Ruelas-Leyva, J.P.; Fuentes, G.A. Chiral Catalyst Deactivation during the Asymmetric Hydrogenation of Acetophenone. Catalysts 2017, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Sui, Z.; Zhou, X.; Chen, D. Kinetics of propane dehydrogenation over Pt–Sn/Al2O3 catalyst. Appl. Catal. A Gen. 2011, 398, 18–26. [Google Scholar] [CrossRef]
- Kaylor, N.; Davis, R.J. Propane dehydrogenation over supported Pt-Sn nanoparticles. J. Catal. 2018, 367, 181–193. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, S.; Goetze, J.; Pletcher, P.; Guo, H.; Kovarik, L.; Artyushkova, K.; Weckhuysen, B.M.; Datye, A.K. Thermally Stable and Regenerable Platinum–Tin Clusters for Propane Dehydrogenation Prepared by Atom Trapping on Ceria. Angew. Chem. Int. Ed. 2017, 56, 8986–8991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuo, Y.-X.; Shi, L.-J.; Cheng, H.-Y.; Zhu, Y.-A.; Yang, M.-L.; Xu, J.; Han, Y.-F.; Li, P.; Yuan, W.-K. Insight into the support effect on the particle size effect of Pt/C catalysts in dehydrogenation. J. Catal. 2018, 360, 175–186. [Google Scholar] [CrossRef]
- Bai, L.Y.; Zhou, Y.M.; Zhang, Y.W.; Liu, H.; Tang, M.H. Influence of Calcium Addition on Catalytic Properties of PtSn/ZSM-5 Catalyst for Propane Dehydrogenation. Catal. Lett. 2009, 129, 449–456. [Google Scholar] [CrossRef]
- Liu, X.; Lang, W.-Z.; Long, L.-L.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic performance in propane dehydrogenation of PtSn/γ-Al2O3 catalysts by doping indium. Chem. Eng. J. 2014, 247, 183–192. [Google Scholar] [CrossRef]
- Ruelas-Leyva, J.P.; Mata-Martinez, A.; Talavera-López, A.; Gómez, S.A.; Jimenez-Lam, S.A.; Fuentes, G.A. Dehydrogenation of Propane to Propylene with Highly Stable Catalysts of Pt-Sn Supported over Mesoporous Silica KIT-6. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Mata-Martinez, A.; Jimenez-Lam, S.A.; Talavera-López, A.; Gómez, S.A.; Fuentes, G.A.; Picos-Corrales, L.A.; Piña-Victoria, J.C.; Ruelas-Leyva, J.P. The Effect of Sn Content in a Pt/KIT-6 Catalyst over its Performance in the Dehydrogenation of Propane. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Naidu Talapaneni, S.; Ramadass, K.; Ruban, S.J.; Benzigar, M.; Lakhi, K.S.; Yang, J.-H.; Ravon, U.; Albahily, K.; Vinu, A. 3D cubic mesoporous C3N4 with tunable pore diameters derived from KIT-6 and their application in base catalyzed Knoevenagel reaction. Catal. Today 2019, 324, 33–38. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Pandurangan, A. Up-gradation of α-tetralone to jet-fuel range hydrocarbons by vapour phase hydrodeoxygenation over PdNi/SBA-16 catalysts. Energy 2017, 140, 1158–1172. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Microporous Mesoporous Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Kim, T.-W.; Ryoo, R.; Kruk, M.; Gierszal, K.P.; Jaroniec, M.; Kamiya, S.; Terasaki, O. Tailoring the Pore Structure of SBA-16 Silica Molecular Sieve through the Use of Copolymer Blends and Control of Synthesis Temperature and Time. J. Phys. Chem. B 2004, 108, 11480–11489. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Chen, D.; Holmen, A.; Walmsley, J.C. Dehydrogenation of propane over Pt-SBA-15 and Pt-Sn-SBA-15: Effect of Sn on the dispersion of Pt and catalytic behavior. Catal. Today 2009, 142, 17–23. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Zhu, Q.; Zhu, X.; Li, X.; Wang, H.; Li, C.; Shan, H. Sn-containing hexagonal mesoporous silica (HMS) for catalytic dehydrogenation of propane: An efficient strategy to enhance stability. J. Catal. 2017, 351, 90–94. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, Y.; Zhang, Y.; Sheng, X.; Zhou, S.; Zhang, Z. Effect of aluminum modification on catalytic properties of PtSn-based catalysts supported on SBA-15 for propane dehydrogenation. J. Nat. Gas Chem. 2012, 21, 207–214. [Google Scholar] [CrossRef]
- Fan, X.; Li, J.; Zhao, Z.; Wei, Y.; Liu, J.; Duan, A.; Jiang, G. Dehydrogenation of propane over PtSnAl/SBA-15 catalysts: Al addition effect and coke formation analysis. Catal. Sci. Technol. 2015, 5, 339–350. [Google Scholar] [CrossRef]
- Shi, L.; Deng, G.-M.; Li, W.-C.; Miao, S.; Wang, Q.-N.; Zhang, W.-P.; Lu, A.-H. Al2O3 Nanosheets Rich in Pentacoordinate Al3+ Ions Stabilize Pt-Sn Clusters for Propane Dehydrogenation. Angew. Chem. Int. Ed. 2015, 54, 13994–13998. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.R.; Wu, J.F.; Jong, S.J.; Tsai, T.C.; Liu, S.B. An ex situ methodology for characterization of coke by TGA and 13C CP-MAS NMR spectroscopy. Appl. Catal. A Gen. 1997, 165, 489–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Qiu, A.; Wang, Y.; Xu, Y.; Wu, P. Effect of Alumina Binder on Catalytic Performance of PtSnNa/ZSM-5 Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2006, 45, 2213–2219. [Google Scholar] [CrossRef]
- Li, Q.; Sui, Z.; Zhou, X.; Zhu, Y.; Zhou, J.; Chen, D. Coke Formation on Pt–Sn/Al2O3 Catalyst in Propane Dehydrogenation: Coke Characterization and Kinetic Study. Top. Catal. 2011, 54, 888. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Chen, D.; Walmsley, J.C.; Holmen, A. Dehydrogenation of propane over Pt-SBA-15: Effect of Pt particle size. Catal. Commun. 2008, 9, 747–750. [Google Scholar] [CrossRef]
- Van Sint Annaland, M.; Kuipers, J.A.M.; van Swaaij, W.P.M. A kinetic rate expression for the time-dependent coke formation rate during propane dehydrogenation over a platinum alumina monolithic catalyst. Catal. Today 2001, 66, 427–436. [Google Scholar] [CrossRef]
- Lobera, M.P.; Téllez, C.; Herguido, J.; Menéndez, M. Transient kinetic modelling of propane dehydrogenation over a Pt–Sn–K/Al2O3 catalyst. Appl. Catal. A Gen. 2008, 349, 156–164. [Google Scholar] [CrossRef]
- Guichard, B.; Roy-Auberger, M.; Devers, E.; Rebours, B.; Quoineaud, A.A.; Digne, M. Characterization of aged hydrotreating catalysts. Part I: Coke depositions, study on the chemical nature and environment. Appl. Catal. A Gen. 2009, 367, 1–8. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Effect of Mg and Ca addition on coke deposition over Cu–Ni/SiO2 catalysts for ethanol steam reforming. Chem. Eng. J. 2010, 163, 395–402. [Google Scholar] [CrossRef]
- Tian, Y.-P.; Liu, X.-M.; Rood, M.J.; Yan, Z.-F. Study of coke deposited on a VOx-K2O/γ-Al2O3 catalyst in the non-oxidative dehydrogenation of isobutane. Appl. Catal. A Gen. 2017, 545, 1–9. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Zhang, Y.; Sheng, X.; Zhang, Z. The synthesis of new coke-resistant support and its application in propane dehydrogenation to propene. J. Chem. Technol. Biotechnol. 2016, 91, 1072–1081. [Google Scholar] [CrossRef]
- Nawaz, Z.; Tang, X.; Wang, Y.; Wei, F. Parametric Characterization and Influence of Tin on the Performance of Pt−Sn/SAPO-34 Catalyst for Selective Propane Dehydrogenation to Propylene. Ind. Eng. Chem. Res. 2010, 49, 1274–1280. [Google Scholar] [CrossRef]
- Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. Propane Dehydrogenation over Pt/TiO2–Al2O3 Catalysts. ACS Catal. 2015, 5, 438–447. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Sattler, J.J.H.B.; Beale, A.M.; Weckhuysen, B.M. Operando Raman spectroscopy study on the deactivation of Pt/Al2O3 and Pt–Sn/Al2O3 propane dehydrogenation catalysts. Phys. Chem. Chem. Phys. 2013, 15, 12095–12103. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zhou, Z.; Shishido, T. Behavior of active species on Pt-Sn/SiO2 catalyst during the dehydrogenation of propane and regeneration. Appl. Catal. A Gen. 2020, 606, 117826. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Sepúlveda-Escribano, A. Low temperature glycerol steam reforming on bimetallic PtSn/C catalysts: On the effect of the Sn content. Fuel 2017, 194, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Juez, A.; Beale, A.M.; Maaijen, K.; Weng, T.C.; Glatzel, P.; Weckhuysen, B.M. A combined in situ time-resolved UV–Vis, Raman and high-energy resolution X-ray absorption spectroscopy study on the deactivation behavior of Pt and PtSn propane dehydrogenation catalysts under industrial reaction conditions. J. Catal. 2010, 276, 268–279. [Google Scholar] [CrossRef]
- Collett, C.H.; McGregor, J. Things go better with coke: The beneficial role of carbonaceous deposits in heterogeneous catalysis. Catal. Sci. Technol. 2016, 6, 363–378. [Google Scholar] [CrossRef]
- Barbier, J. Deactivation of reforming catalysts by coking—A review. Appl. Catal. 1986, 23, 225–243. [Google Scholar] [CrossRef]
- Wolf, M.; Raman, N.; Taccardi, N.; Haumann, M.; Wasserscheid, P. Coke Formation during Propane Dehydrogenation over Ga−Rh Supported Catalytically Active Liquid Metal Solutions. ChemCatChem 2020, 12, 1085–1094. [Google Scholar] [CrossRef]
- Kleitz, F.; Czuryszkiewicz, T.; Solovyov, L.A.; Lindén, M. X-ray Structural Modeling and Gas Adsorption Analysis of Cagelike SBA-16 Silica Mesophases Prepared in a F127/Butanol/H2O System. Chem. Mater. 2006, 18, 5070–5079. [Google Scholar] [CrossRef]
- Gobin, O.C.; Wan, Y.; Zhao, D.; Kleitz, F.; Kaliaguine, S. Mesostructured silica SBA-16 with tailored intrawall porosity part 1: Synthesis and characterization. J. Phys. Chem. C 2007, 111, 3053–3058. [Google Scholar] [CrossRef]













| Sample | Nominal Pt/Sn (wt%/wt%) | Real Pt/Sn (wt%/wt%) | HTT a (°C) | SBET (m2 g−1) | Dp (nm) | Vp (cm3 g−1) |
|---|---|---|---|---|---|---|
| SBA-60 | 0/0 | ND b | 60 | 453 | 1.4 | 0.30 |
| SBA-100 | 0/0 | ND b | 100 | 967 | 1.8 | 0.84 |
| SBA-140 | 0/0 | ND b | 140 | 572 | 4.0 | 1.18 |
| 0.5-Pt-0-Sn/SBA-100 | 0.5/0 | 0.30/0.00 | 100 | 894 | 1.8 | 0.78 |
| 0.5-Pt-1-Sn/SBA-60 | 0.5/1 | 0.30/0.88 | 60 | 433 | 1.3 | 0.29 |
| 0.5-Pt-1-Sn/SBA-100 | 0.5/1 | 0.22/0.85 | 100 | 956 | 1.7 | 0.81 |
| 0.5-Pt-1-Sn/SBA-140 | 0.5/1 | 0.22/0.81 | 140 | 561 | 4.0 | 1.12 |
| 0.5-Pt-2-Sn/SBA-100 | 0.5/2 | 0.26/1.50 | 100 | 690 | 1.7 | 0.60 |
| TOS (min) | Coke Content (wt%) Considering the Reduced Sample | Coke Content (wt%) without Considering the Reduced Sample | ||||
|---|---|---|---|---|---|---|
| Soft Coke | Hard Coke | Total | Soft Coke | Hard Coke | Total | |
| 180–330 °C | 330–700 °C | 180–330 °C | 330–700 °C | |||
| 0 | 0.89 | 2.25 | 3.14 | 0.00 | 0.00 | 0.00 |
| 60 | 1.27 | 2.72 | 3.99 | 0.38 | 0.47 | 0.85 |
| 180 | 1.32 | 3.12 | 4.44 | 0.43 | 0.87 | 1.30 |
| 300 | 1.22 | 3.92 | 5.14 | 0.33 | 1.67 | 2.00 |
| TOS (min) | ID/IG | ΓG (cm−1) | Position (cm−1) | |
|---|---|---|---|---|
| IG | ID | |||
| 0 | ND * | ND * | ND * | ND * |
| 60 | 3.85 | 50.65 | 1605 | 1336 |
| 180 | 2.22 | 54.55 | 1600 | 1335 |
| 300 | 3.66 | 38.15 | 1598 | 1332 |
| TOS (min) | Pt alloy Particle Size (nm) |
|---|---|
| 0 | 7.13 |
| 180 | 8.16 |
| 300 | 20.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruelas-Leyva, J.P.; Maldonado-Garcia, L.F.; Talavera-Lopez, A.; Santos-López, I.A.; Picos-Corrales, L.A.; Santolalla-Vargas, C.E.; Torres, S.A.G.; Fuentes, G.A. A Comprehensive Study of Coke Deposits on a Pt-Sn/SBA-16 Catalyst during the Dehydrogenation of Propane. Catalysts 2021, 11, 128. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010128
Ruelas-Leyva JP, Maldonado-Garcia LF, Talavera-Lopez A, Santos-López IA, Picos-Corrales LA, Santolalla-Vargas CE, Torres SAG, Fuentes GA. A Comprehensive Study of Coke Deposits on a Pt-Sn/SBA-16 Catalyst during the Dehydrogenation of Propane. Catalysts. 2021; 11(1):128. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010128
Chicago/Turabian StyleRuelas-Leyva, Jose P., Luis F. Maldonado-Garcia, Alfonso Talavera-Lopez, Iván A. Santos-López, Lorenzo A. Picos-Corrales, Carlos E. Santolalla-Vargas, Sergio A. Gómez Torres, and Gustavo A. Fuentes. 2021. "A Comprehensive Study of Coke Deposits on a Pt-Sn/SBA-16 Catalyst during the Dehydrogenation of Propane" Catalysts 11, no. 1: 128. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010128