Novel Preparation of Cu and Fe Zirconia Supported Catalysts for Selective Catalytic Reduction of NO with NH3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. Structural and Textural Properties of ZrO2 Supported Catalysts
2.1.2. Acidic Properties of ZrO2 Supported Catalysts
2.1.3. Surface Properties of ZrO2 Supported Catalysts
2.2. Catalytic Results
2.2.1. NH3-SCR of NO in the Absence and Presence of Water Vapor
2.2.2. NH3-SCO in the Absence and Presence of Water Vapor
2.2.3. SO2 Activation of Cu-Zr and Fe-Zr Catalysts and their Catalytic Behavior in NH3-SCR of NO in the Absence and Presence of Water Vapor
SO2 Activation of Cu-Zr Catalyst in NH3-SCR of NO in the Absence of Water Vapor
SO2 Activation of Fe-Zr in NH3-SCR of NO in the Presence of Water Vapor
3. Materials and Methods
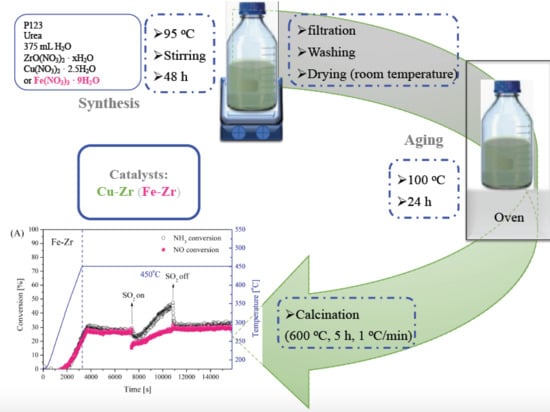
3.1. Catalysts Synthesis
3.2. Characterization Techniques
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vascellari, M. NOx Emission and Mitigation Technologies. In Handbook of Clean Energy Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–23. ISBN 9781118991978. [Google Scholar]
- Iwamoto, M.; Furukawa, H.; Mine, Y.; Uemura, F.; Mikuriya, S.I.; Kagawa, S. Copper(II) Ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. J. Chem. Soc. Ser. Chem. Commun. 1986, 1272–1273. [Google Scholar] [CrossRef]
- Ishihara, T.; Ando, M.; Sada, K.; Takiishi, K.; Yamada, K.; Nishiguchi, H.; Takita, Y. Direct decomposition of NO into N2 and O2 over La(Ba)Mn(In)O3 perovskite oxide. J. Catal. 2003, 220, 104–114. [Google Scholar] [CrossRef]
- Liu, G.; Gao, P.X. A review of NOx storage/reduction catalysts: Mechanism, materials and degradation studies. Catal. Sci. Technol. 2011, 1, 552–568. [Google Scholar] [CrossRef]
- Seo, C.K.; Kim, H.; Choi, B.; Lim, M.T. The optimal volume of a combined system of LNT and SCR catalysts. J. Ind. Eng. Chem. 2011, 17, 382–385. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R. A review of the effect of the addition of hydrogen in the selective catalytic reduction of NOx with hydrocarbons on silver catalysts. Top. Catal. 2006, 39, 53–58. [Google Scholar] [CrossRef]
- Chen, G.; Xu, J.; Yu, H.; Guo, F.; Xie, J.; Wang, Y. Effect of the non-thermal plasma treatment on the structure and SCR activity of vanadium-based catalysts. Chem. Eng. J. 2020, 380, 122286. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Święs, A.; Gil, B.; Rutkowska, M.; Piwowarska, Z.; Borcuch, A.; Michalik, M.; Chmielarz, L. Effective catalysts for the low-temperature NH3-SCR process based on MCM-41 modified with copper by template ion-exchange (TIE) method. Appl. Catal. B Environ. 2018, 237, 927–937. [Google Scholar] [CrossRef]
- Abid, R.; Delahay, G.; Tounsi, H. Selective catalytic reduction of NO by NH3 on cerium modified faujasite zeolite prepared from aluminum scraps and industrial metasilicate. J. Rare Earths 2020, 38, 250–256. [Google Scholar] [CrossRef]
- Kieffer, C.; Lavy, J.; Jeudy, E.; Bats, N.; Delahay, G. Characterisation of a commercial automotive NH3-SCR copper-zeolite catalyst. Top. Catal. 2013, 56, 40–44. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. A new V2O5-MoO3-TiO2-SO42− nanostructured aerogel catalyst for diesel DeNOx technology. New J. Chem. 2020, 44, 16119–16134. [Google Scholar] [CrossRef]
- Leistner, K.; Mihai, O.; Wijayanti, K.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions. Catal. Today 2015, 258, 49–55. [Google Scholar] [CrossRef]
- Jabłońska, M.; Delahay, G.; Kruczała, K.; Błachowski, A.; Tarach, K.A.; Brylewska, K.; Petitto, C.; Góra-Marek, K. Standard and fast selective catalytic reduction of NO with NH3 on zeolites Fe-BEA. J. Phys. Chem. C 2016, 120, 16831–16842. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Li, D.; Qin, Y.; Xie, Y.; Wang, S. WO3/CeO2-ZrO2 a promising catalyst for selective catalytic reduction (SCR) of NOx with NH3 in diesel exhaust. Chem. Commun. 2008, 1470–1472. [Google Scholar] [CrossRef]
- Djerad, S.; Geiger, B.; Schott, F.J.P.; Kureti, S. Synthesis of nano-sized ZrO2 and its use as catalyst support in SCR. Catal. Commun. 2009, 10, 1103–1106. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.T.; Kureti, S.; Reichert, D.; Schott, F.; Weisweiler, W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts. Appl. Catal. B Environ. 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Ma, H.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Peng, B.; Rappé, K.G.; Cui, Y.; Gao, F.; Szanyi, J.; Olszta, M.J.; Walter, E.D.; Wang, Y.; Holladay, J.D.; Goffe, R.A. Enhancement of high-temperature selectivity on Cu-SSZ-13 towards NH3-SCR reaction from highly dispersed ZrO2. Appl. Catal. B Environ. 2020, 263. [Google Scholar] [CrossRef]
- Verdier, S.; Rohart, E.; Bradshaw, H.; Harris, D.; Bichon, P.; Delahay, G. Acidic zirconia materials for durable NH3-SCR deNOx catalysts. SAE Tech. Pap. 2008, 1, 1022. [Google Scholar]
- Delahay, G.; Ensuque, E.; Coq, B.; Figuéras, F. Selective catalytic reduction of nitric oxide by n-decane on Cu/sulfated-zirconia catalysts in oxygen rich atmosphere: Effect of sulfur and copper contents. J. Catal. 1998, 175, 7–15. [Google Scholar] [CrossRef]
- Delahay, G.; Coq, B.; Ensuque, E.; Figuéras, F. Catalytic behaviour of Cu/ZrO2 and Cu/ZrO2(SO42−) in the reduction of nitric oxide by decane in oxygen-rich atmosphere. Catal. Lett. 1996, 39, 105–109. [Google Scholar] [CrossRef]
- Figueras, F.; Coq, B.; Ensuque, E.; Tachon, D.; Delahay, G. Catalytic properties of Cu on sulphated zirconias for DeNOx in excess of oxygen using n-decane as reductant. Catal. Today 1998, 42, 117–125. [Google Scholar] [CrossRef]
- Pasel, J.; Speer, V.; Albrecht, C.; Richter, F.; Papp, H. Metal doped sulfated ZrO2 as catalyst for the selective catalytic reduction (SCR) of NO with propane. Appl. Catal. B Environ. 2000, 25, 105–113. [Google Scholar] [CrossRef]
- Pietrogiacomi, D.; Sannino, D.; Magliano, A.; Ciambelli, P.; Tuti, S.; Indovina, V. The catalytic activity of CuSO4/ZrO2 for the selective catalytic reduction of NOx with NH3 in the presence of excess O2. Appl. Catal. B Environ. 2002, 36, 217–230. [Google Scholar] [CrossRef]
- Pietrogiacomi, D.; Magliano, A.; Sannino, D.; Campa, M.C.; Ciambelli, P.; Indovina, V. In situ sulphated CuOx/ZrO2 and CuOx/sulphated-ZrO2 as catalysts for the reduction of NOx with NH3 in the presence of excess O2. Appl. Catal. B Environ. 2005, 60, 83–92. [Google Scholar] [CrossRef]
- Ismail, R.; Arfaoui, J.; Ksibi, Z.; Ghorbel, A.; Delahay, G. Effect of the iron amount on the physicochemical properties of Fe–ZrO2 aerogel catalysts for the total oxidation of toluene in the presence of water vapor. J. Porous Mater. 2020, 27, 1847–1852. [Google Scholar] [CrossRef]
- Navío, J.A.; Hidalgo, M.C.; Colón, G.; Botta, S.G.; Litter, M.I. Preparation and physicochemical properties of ZrO2 and Fe/ZrO2 prepared by a sol-gel technique. Langmuir 2001, 17, 202–210. [Google Scholar] [CrossRef]
- López-Hernández, I.; Mengual, J.; Palomares, A.E. The influence of the support on the activity of Mn–Fe catalysts used for the selective catalytic reduction of NOx with ammonia. Catalysts 2020, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Kustov, A.L.; Rasmussen, S.B.; Fehrmann, R.; Simonsen, P. Activity and deactivation of sulphated TiO2- and ZrO2-based V, Cu, and Fe oxide catalysts for NO abatement in alkali containing flue gases. Appl. Catal. B Environ. 2007, 76, 9–14. [Google Scholar] [CrossRef]
- Indovina, V.; Campa, M.C.; Pepe, F.; Pietrogiacomi, D.; Tuti, S. Iron species in FeOx/ZrO2 and FeOx/sulphated-ZrO2 catalysts. Stud. Surf. Sci. Catal. 2005, 155, 329–337. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, Z.; Liu, C.; Liu, Q. Investigation of sulfated iron-based catalysts with different sulfate position for selective catalytic reduction of NOx with NH3. Catalysts 2020, 10, 1035. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Bi, Y.; Zhang, Z. A study on the selective catalytic reduction of NOx: by ammonia on sulphated iron-based catalysts. RSC Adv. 2020, 10, 40948–40959. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, B.; Zhou, H.; Feng, C.; Wang, X.; Yuan, K.; Gan, X.; Zhu, L.; Zhang, G.; Xu, D. Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent. Appl. Surf. Sci. 2017, 399, 288–297. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Wang, H.; Mo, J.; Wu, Z.; Liu, Y.; Weng, X. Ceria supported on sulfated zirconia as a superacid catalyst for selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2013, 394, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kogler, M.; Köck, E.M.; Vanicek, S.; Schmidmair, D.; Götsch, T.; Stöger-Pollach, M.; Hejny, C.; Klötzer, B.; Penner, S. Enhanced kinetic stability of pure and Y-doped tetragonal ZrO2. Inorg. Chem. 2014, 53, 13247–13257. [Google Scholar] [CrossRef]
- Tan, D.; Lin, G.; Liu, Y.; Teng, Y.; Zhuang, Y.; Zhu, B.; Zhao, Q.; Qiu, J. Synthesis of nanocrystalline cubic zirconia using femtosecond laser ablation. J. Nanoparticle Res. 2011, 13, 1183–1190. [Google Scholar] [CrossRef]
- Boroń, P.; Chmielarz, L.; Dzwigaj, S. Influence of Cu on the catalytic activity of FeBEA zeolites in SCR of NO with NH3. Appl. Catal. B Environ. 2015, 168–169, 377–384. [Google Scholar] [CrossRef]
- Du, L.; Wang, W.; Yan, H.; Wang, X.; Jin, Z.; Song, Q.; Si, R.; Jia, C. Copper-ceria sheets catalysts: Effect of copper species on catalytic activity in CO oxidation reaction. J. Rare Earths 2017, 35, 1186–1196. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Breviglieri, S.T.; Cavalheiro, É.T.G.; Chierice, G.O. Correlation between ionic radius and thermal decomposition of Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) diethanoldithiocarbamates. Thermochim. Acta 2000, 356, 79–84. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Wang, Y.; Hu, C.; Da Costa, P. One-Step synthesis of highly active and stable Ni-ZrOx for dry reforming of methane. Ind. Eng. Chem. Res. 2020, 59, 11441–11452. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ismail, R.; Arfaoui, J.; Ksibi, Z.; Ghorbel, A.; Delahay, G. Ag/ZrO2 and Ag/Fe–ZrO2 catalysts for the low temperature total oxidation of toluene in the presence of water vapor. Transit. Met. Chem. 2020, 45, 501–509. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2-TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2012, 195–196, 323–331. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Wang, Y.; Cao, T.; Zhao, G. Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B Environ. 2012, 125, 120–127. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Da Costa, P.; Hu, C. Highly carbon-resistant Y doped NiO–ZrOm catalysts for dry reforming of methane. Catalysts 2019, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C-H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, J.; Wang, C.; Li, Y.; Chen, Y.; Zhang, M. Effect of cosolvent and temperature on the structures and properties of Cu-MOF-74 in low-temperature NH3-SCR. Ind. Eng. Chem. Res. 2017, 56, 3542–3550. [Google Scholar] [CrossRef]
- Khodayari, R.; Odenbrand, C.U.I. Regeneration of commercial SCR catalysts by washing and sulphation: Effect of sulphate groups on the activity. Appl. Catal. B Environ. 2001, 33, 277–291. [Google Scholar] [CrossRef]
- Miao, J.; Li, H.; Su, Q.; Yu, Y.; Chen, Y.; Chen, J.; Wang, J. The combined promotive effect of SO2 and HCl on Pb-poisoned commercial NH3-SCR V2O5-WO3/TiO2 catalysts. Catal. Commun. 2019, 125, 118–122. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Chen, J.; Meng, X.; Chen, Y.; He, C. Promotive effect of SO2 on the activity of a deactivated commercial selective catalytic reduction ratalyst: An in situ DRIFT study. Ind. Eng. Chem. Res. 2014, 53, 16229–16234. [Google Scholar] [CrossRef]








| Sample | Bragg Angles/° | d-Spacing/Å | Crystallite Size of ZrO2/nm * | Specific Surface Area/m2/g | Volume of Mesopores/cm3/g | Average Pore Size/nm |
|---|---|---|---|---|---|---|
| ZrO2 | 30.192 | 2.9576 | 13.7 | 81 | 0.12 | 5.0 |
| Cu-Zr | 30.376 | 2.9402 | 11.3 | 128 | 0.06 | 3.3 |
| Fe-Zr | 30.349 | 2.9427 | 8.8 | 139 | 0.09 | 3.5 |
| Sample | Zr (wt%) | O (wt%) | Cu (wt%) | Fe (wt%) | O Species (%) | Zr Species (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| O2− | CO32− | OH− | Zr3+ | Zr4+ | |||||
| ZrO2 | 66.8 | 33.2 | - | - | 70.6 | 18.1 | 11.3 | 37.5 | 62.5 |
| Cu-Zr | 66.6 | 31.2 | 2.2 | - | 64.9 | 24.7 | 10.4 | 42.8 | 57.2 |
| Fe-Zr | 64.4 | 32.4 | - | 3.2 | 75.8 | 19.9 | 4.3 | 95.3 | 4.7 |
| Sample | P123/g | Urea/g | ZrO(NO)3·2H2O/g | Cu(NO)2·2.5H2O/g | Fe(NO)3·9H2O/g |
|---|---|---|---|---|---|
| Cu-Zr | 7.84 | 7.50 | 5.03 | 0.14 | - |
| Fe-Zr | 7.84 | 7.50 | 5.03 | - | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świrk, K.; Wang, Y.; Hu, C.; Li, L.; Da Costa, P.; Delahay, G. Novel Preparation of Cu and Fe Zirconia Supported Catalysts for Selective Catalytic Reduction of NO with NH3. Catalysts 2021, 11, 55. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010055
Świrk K, Wang Y, Hu C, Li L, Da Costa P, Delahay G. Novel Preparation of Cu and Fe Zirconia Supported Catalysts for Selective Catalytic Reduction of NO with NH3. Catalysts. 2021; 11(1):55. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010055
Chicago/Turabian StyleŚwirk, Katarzyna, Ye Wang, Changwei Hu, Li Li, Patrick Da Costa, and Gérard Delahay. 2021. "Novel Preparation of Cu and Fe Zirconia Supported Catalysts for Selective Catalytic Reduction of NO with NH3" Catalysts 11, no. 1: 55. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010055