Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia coli Inactivation in Water
Abstract
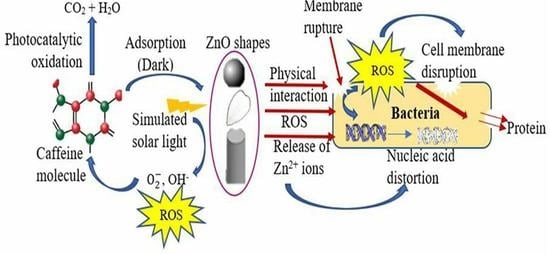
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
2.1.1. Electron Microscopy
2.1.2. BET
2.1.3. X-ray Diffraction (XRD)
2.1.4. Zeta Potential and Zn2+ Generation
2.1.5. Generation of Hydroxyl (OH•) and Superoxide Radicals (•O2−)
2.2. Mechanism of Morphology Change with the Choice of Solvent
2.3. Caffeine Degradation
2.3.1. Adsorption Study
2.3.2. Photocatalytic Experiments
Nanoparticle Dosage
Initial Concentration of Caffeine and Nanoparticle Morphology
Light Intensity
2.4. Antibacterial Activity
2.4.1. Minimum Inhibitory Concentration (MIC)
2.4.2. Zone of Inhibition (Disc Diffusion Assay)
2.4.3. Cell Viability Assay (CFU/mL)
2.4.4. Protein Leakage Analysis
2.4.5. Imaging
3. Experimental Details
3.1. Materials
3.2. Method
3.2.1. Synthesis of Nanoparticles
3.2.2. Characterization of Nanomaterials
3.2.3. Photocatalytic Degradation of Caffeine
Adsorption Study
Kinetic Study
3.2.4. Bacterial Toxicity Assessment
Minimum Inhibitory Concentration (MIC)
Zone of Inhibition
Cell Viability Assay (CFU/mL)
Protein Leakage Analysis (Bradford Assay)
Imaging of Bacteria-Nanoparticle Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476. [Google Scholar] [CrossRef]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Envrion. Sci. Pollut. Res. 2020, 27, 34715. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Griffin, P.M.; Tauxe, R.V. The Epidemiology of Infections Caused by Escherichia coli O157:H7, Other Enterohemorrhagic E. coli, and the Associated Hemolytic Uremic Syndrome. Epidemiol. Rev. 1991, 13, 60. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Effic. Technol. 2016, 2, 175. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandal, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Denga, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanak, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; p. 425. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69. [Google Scholar] [CrossRef]
- Muggli, D.S.; Ding, L. Photocatalytic performance of sulfated TiO2 and Degussa P-25 TiO2 during oxidation of organics. Appl. Catal. B 2001, 32, 181. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative ecotoxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527. [Google Scholar] [CrossRef]
- Li, G.R.; Hu, T.; Pan, G.L.; Yan, T.Y.; Gao, X.P.; Zhu, H.Y. Morphology−Function Relationship of ZnO: Polar Planes, Oxygen Vacancies, and Activity. J. Phys. Chem. C 2008, 112, 11859. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66. [Google Scholar] [CrossRef]
- Thakur, S.; Neogi, S. Effect of doped ZnO nanoparticles on bacterial cell morphology and biochemical composition. Appl. Nanosci. 2020. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundrarajan, M.; Ambik, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294. [Google Scholar] [CrossRef]
- Arfanis, M.K.; Adamou, P.; Moustakas, N.G.; Triantis, T.M.; Kontos, A.G.; Falaras, P. Photocatalytic degradation of salicylic acid and caffeine emerging contaminants using titania nanotubes. Chem. Eng. J. 2017, 310, 525. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environmental Nanotechnology. Monit. Manag. 2018, 10, 63. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O. UV-LEDs floating-bed photoreactor for the removal of caffeine and paracetamol using ZnO supported on polystyrene pellets. Chem. Eng. J. 2018, 350, 703. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55. [Google Scholar] [CrossRef]
- Barnes, R.J.; Molina, R.; Xu, J.; Dobson, P.J.; Thompson, I.P. Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J. Nanopart. Res. 2013, 15, 1432. [Google Scholar] [CrossRef]
- Singh, R.; Verma, K.; Patyal, A.; Sharma, I.; Barman, P.B.; Sharma, D. Nanosheet and nanosphere morphology dominated photocatalytic & antibacterial properties of ZnO nanostructures. Solid State Sci. 2019, 89, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Nann, T. Shape Control of II–VI Semiconductor Nanomaterials. Small 2006, 2, 316. [Google Scholar] [CrossRef] [PubMed]
- Achouri, F.; Merlin, C.; Corbel, S.; Alem, H.; Mathieu, L.; Balan, L.; Medjahdi, G.; Said, M.B.; Ghrabi, A.; Schneider, R. ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation. Materials 2018, 11, 2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, S.; Mandal, S.K. Morphology engineering of ZnO nanorod arrays to hierarchical nanoflowers for enhanced photocatalytic activity and antibacterial action against Escherichia coli. New J. Chem. 2020, 44, 11796. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.H.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems—With special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603. [Google Scholar] [CrossRef]
- Kosmulski, M.; Prochniak, P.; Rosenholm, J. Letter: The role of carbonate-free Neodymium (III) oxide. J. Dispers. Sci. Technol. 2009, 30, 589. [Google Scholar] [CrossRef]
- Liao, D.; Wu, G.; Liao, B.-Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Phys. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Saran, M.; Summer, K.H. Assaying for hydroxyl radicals: Hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic. Res. 1999, 31, 429–436. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Ayudhya, S.K.N.; Tonto, P.; Mekasuwandumrong, O.; Pavarajarn, V.; Praserthdam, P. Solvothermal Synthesis of ZnO with Various Aspect Ratios Using Organic Solvents. Cryst. Growth Des. 2006, 6, 2446. [Google Scholar] [CrossRef]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (Chitosan-zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86. [Google Scholar] [CrossRef] [PubMed]
- Thongam, D.D.; Gupta, J.; Sahu, N.K.; Bahadur, D. Investigating the role of different reducing agents, molar ratios, and synthesis medium over the formation of ZnO nanostructures and their photo-catalytic activity. J. Mater. Sci. 2018, 53, 1110–1122. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Antibacterial effect of chronic exposure of low concentration ZnO nanoparticles on E. coli. J. Envrion. Sci. Health Part A 2013, 48, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.M.; Mandal, B.K.; Naidu, E.A.; Sinha, M.; Kumar, K.S.; Reddy, P.S. Synthesis and characterisation of flower shaped Zinc Oxide nanostructures and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 171–174. [Google Scholar] [CrossRef]
- Amna, T. Shape-controlled synthesis of three-dimensional zinc oxide nanoflowers for disinfection of food pathogens. Z. Für Nat. C 2018, 73, 297–301. [Google Scholar] [CrossRef]
- Salah, N.; Al-Shawafi, W.M.; Alshahrie, A.; Baghdadi, N.; Soliman, Y.M.; Memic, A. Size controlled, antimicrobial ZnO nanostructures produced by the microwave assisted route. Mater. Sci. Eng. C 2019, 99, 1164–1173. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Sangkanu, S.; Sukhoom, A.; Muensit, N. Morphology, photocatalytic and antibacterial activities of radial spherical ZnO nanorods controlled with a diblock copolymer. Superlattices Microstruct. 2012, 51, 103–113. [Google Scholar] [CrossRef]
- Smith, A.; McCann, M.; Kavanagh, K. Proteomic analysis of the proteins released from Staphylococcus aureus following exposure to Ag(I). Toxicol. In Vitro 2013, 27, 1644–1648. [Google Scholar] [CrossRef] [Green Version]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Envrion. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, A.M.F.; Fiévet, F. Toxicological Impact Studies Based onEscherichiacoliBacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Thakur, S.; Neogi, S.; Dhara, S. Carbon nano dot decorated copper nanowires for SERS-Fluorescence dual-mode imaging/anti-microbial activity and enhanced angiogenic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117669. [Google Scholar] [CrossRef] [PubMed]







| Particle Size (nm) | Model Pollutant | Photocatalytic Activity (% Degradation) | Maximum %Reduction in E. coli Growth | Ref | |
|---|---|---|---|---|---|
| ZnO sphere | 9.6–25.5 | Methylene blue (MB) | 82.1% at 180 min UV exposure | 69.2% at 100 µg/mL | [20] |
| 133.7–260.2 | MB | 18% and 29% after 1 h UV exposure (1 g/L) | 30 and 35% after 1 h exposure (1 g/L) | [21] | |
| 53.99 | MB | 95.45% after 180 min | Not reported | [22] | |
| 4.35 | 4-nitrophenol | 78% in 100 min | 85% in 5 h at 100 μg/mL | [13] | |
| 65.00 | Acid Orange 74 | 80% after 80 min | 99.93% at 20 min at 20 ppm NP | [23] | |
| ZnO petals | 214.38 × 178.22 | MB | 96.52 after 180 min | Not reported | [22] |
| 45.0 | Acid Orange 74 | 90% after 80 min | 99.97% after 20 min at 20 ppm NP | [23] | |
| (1.41–1.8) × (0.33–0.4) | methylene blue and Congo red | 81% for CR and 67% for MB after 80 min | 90% for 150 mg/mL after 6 h | [24] | |
| ZnO rod | 155.0 | MB | 87.12 after 180 min | Not reported | [22] |
| 20.0 | Orange II | 100% after 150 minsolar irradiation with 1 mg/mL | 100% in >3 h (1 mg/mL) | [25] | |
| 76.0 | Acid Orange 74 | 70% after 80 min | 99.8% after 20 min t 20 ppm NP | [23] |
| Sample | Average Particle Diameter (nm) (TEM) | BET Specific Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Diameter (nm) | Point of Zero Charge |
|---|---|---|---|---|---|
| ZnO sphere | 10.18 | 92.22 | 0.15 | 6.64 | 4.90 |
| ZnO petal | 31.85 (petal thickness) | 12.02 | 0.03 | 10.70 | 6.00 |
| ZnO rod | 157.00 (diameter) | 6.60 | 0.017 | 10.26 | 6.80 |
| Sample | Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | χ2 | 1/n | Kf (mg1−1/n (L) 1/n(g)−1) | R2 | χ2 | |
| ZnO-sphere | 12.57 | 0.06 | 0.89 | 0.61 | 0.45 | 1.74 | 0.80 | 0.85 |
| ZnO-petal | 5.28 | 0.14 | 0.78 | 0.14 | 0.28 | 1.64 | 0.77 | 0.14 |
| ZnO-rod | 6.04 | 0.09 | 0.78 | 0.26 | 0.36 | 1.26 | 0.64 | 0.44 |
| Sample | Parameter | Caffeine Concentration (ppm) | Light Intensity (mW/cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 50 | 150 | 250 | ||
| ZnO sphere | 0.1 k1 * (min−1) | 1.32 | 1.33 | 0.69 | 0.48 | 0.43 | 0.21 | 0.23 | 0.48 |
| R2 | 0.97 | 0.97 | 0.96 | 0.96 | 0.98 | 0.97 | 0.99 | 0.99 | |
| 0.01 k2 * (g/mg min) | 1.74 | 0.97 | 0.26 | 0.16 | 0.10 | 0.07 | 0.06 | 0.09 | |
| R2 | 0.94 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.99 | 0.99 | |
| ZnO petal | 0.1 k1 (min−1) | 1.24 | 0.61 | 0.59 | 0.24 | 0.32 | 0.11 | 0.26 | 0.43 |
| R2 | 0.99 | 0.95 | 0.96 | 0.98 | 0.97 | 0.99 | 0.95 | 0.97 | |
| 0.01 k2 (g/mg min) | 1.65 | 0.34 | 0.22 | 0.05 | 0.07 | 0.03 | 0.05 | 0.07 | |
| R2 | 0.98 | 0.97 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.99 | |
| ZnO rod | 0.1 k1 (min−1) | 0.81 | 0.35 | 0.15 | 0.12 | 0.16 | 0.10 | 0.03 | 0.12 |
| R2 | 0.96 | 0.98 | 0.98 | 0.98 | 0.99 | 0.96 | 0.99 | 0.99 | |
| 0.01 k2 (g/mg min) | 0.77 | 0.15 | 0.03 | 0.02 | 0.04 | 0.02 | 0.01 | 0.02 | |
| R2 | 0.96 | 0.99 | 0.98 | 0.99 | 0.99 | 0.96 | 0.99 | 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, S.; Neogi, S.; Ray, A.K. Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia coli Inactivation in Water. Catalysts 2021, 11, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010063
Thakur S, Neogi S, Ray AK. Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia coli Inactivation in Water. Catalysts. 2021; 11(1):63. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010063
Chicago/Turabian StyleThakur, Shaila, Sudarsan Neogi, and Ajay K. Ray. 2021. "Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia coli Inactivation in Water" Catalysts 11, no. 1: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010063