Iridium Oxide Enabled Sensors Applications
Abstract
:1. Introduction
2. Iridium Oxide Deposition Method
2.1. Anodically Oxidized Iridium Oxide
2.2. Thermal Deposition to Prepare Iridium Oxide
2.3. Sputter-Coating to Deposit Iridium Oxide
2.4. Melt-Oxidized Iridium Oxide
2.5. Iridium Oxide Colloid Formation
3. Iridium Oxide Enabled Sensors
3.1. Glucose Sensor
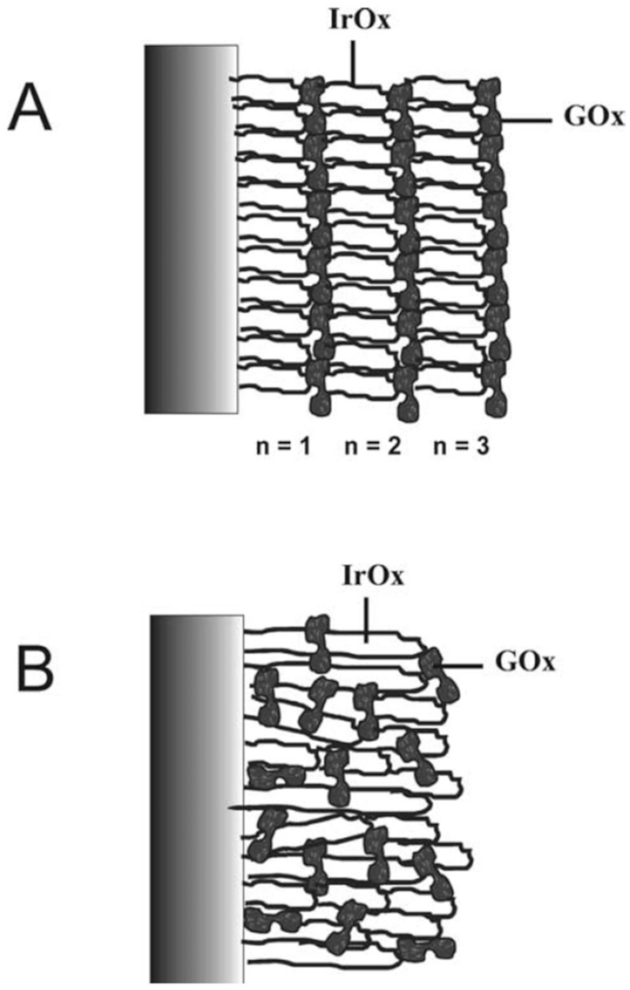
3.1.1. Glucose Oxidase Based IrOx Sensor
3.1.2. Iridium Oxides Nanomaterials for Direct Glucose Oxidation
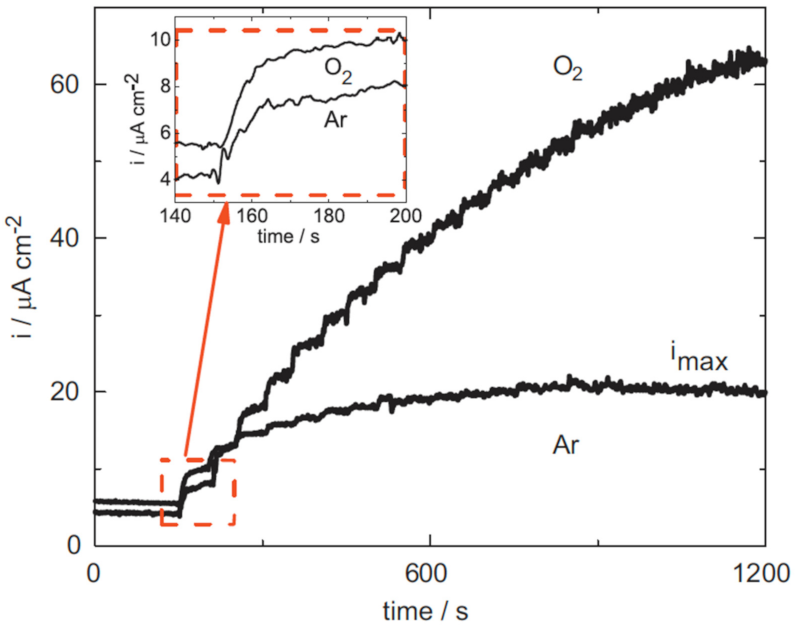
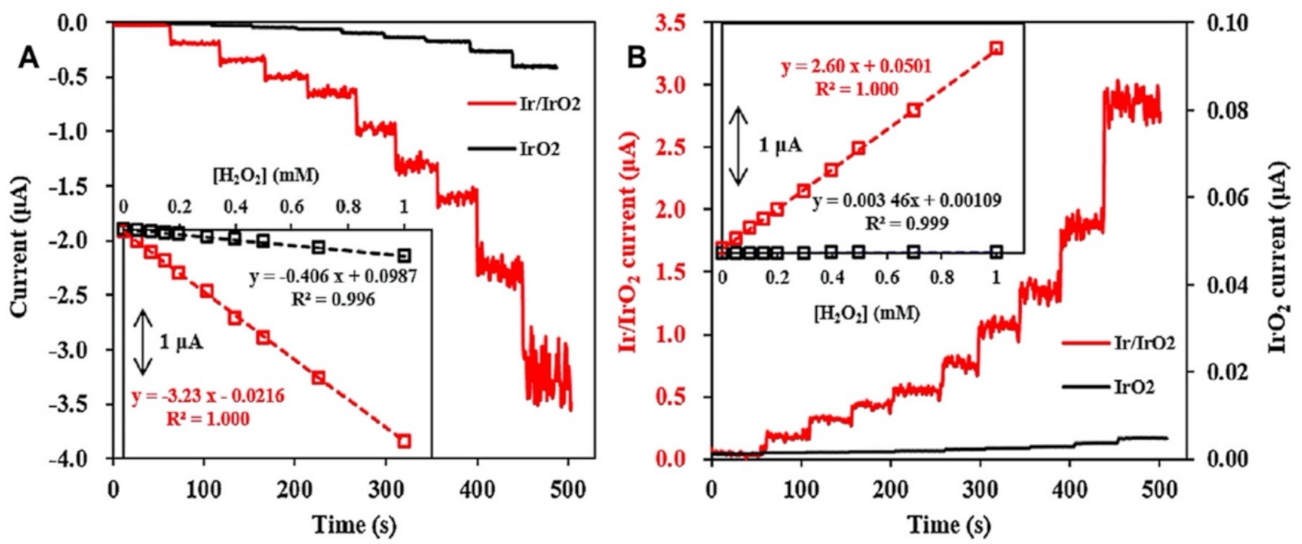
3.2. Hydrogen Peroxide Detection
3.3. pH Sensing of IrOx Applied Materials
3.3.1. pH Sensing of IrOx in the Extracellular Environment
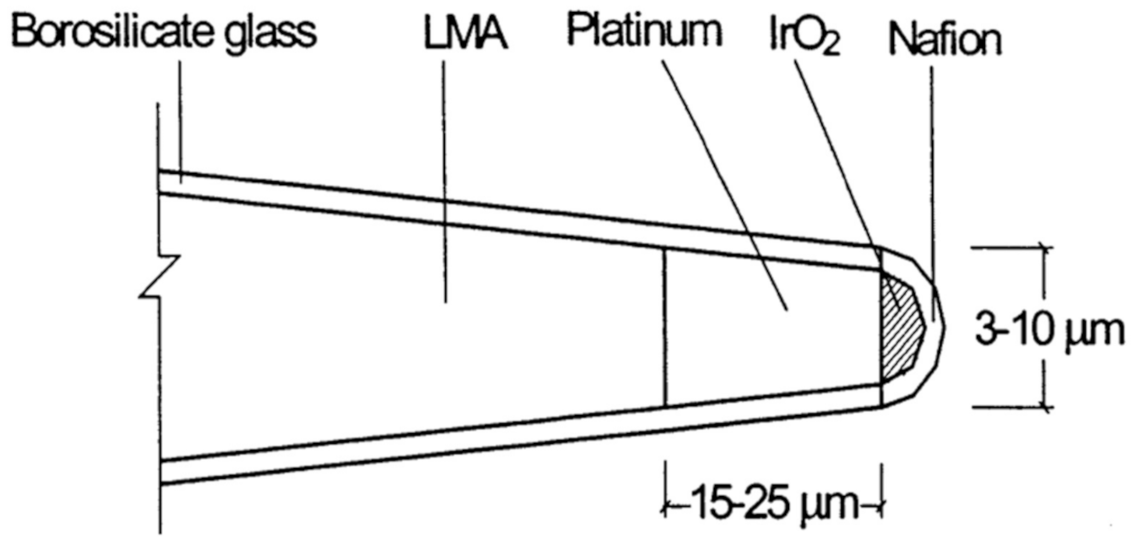
3.3.2. Fiber-Based pH Sensing for In Vivo Applications
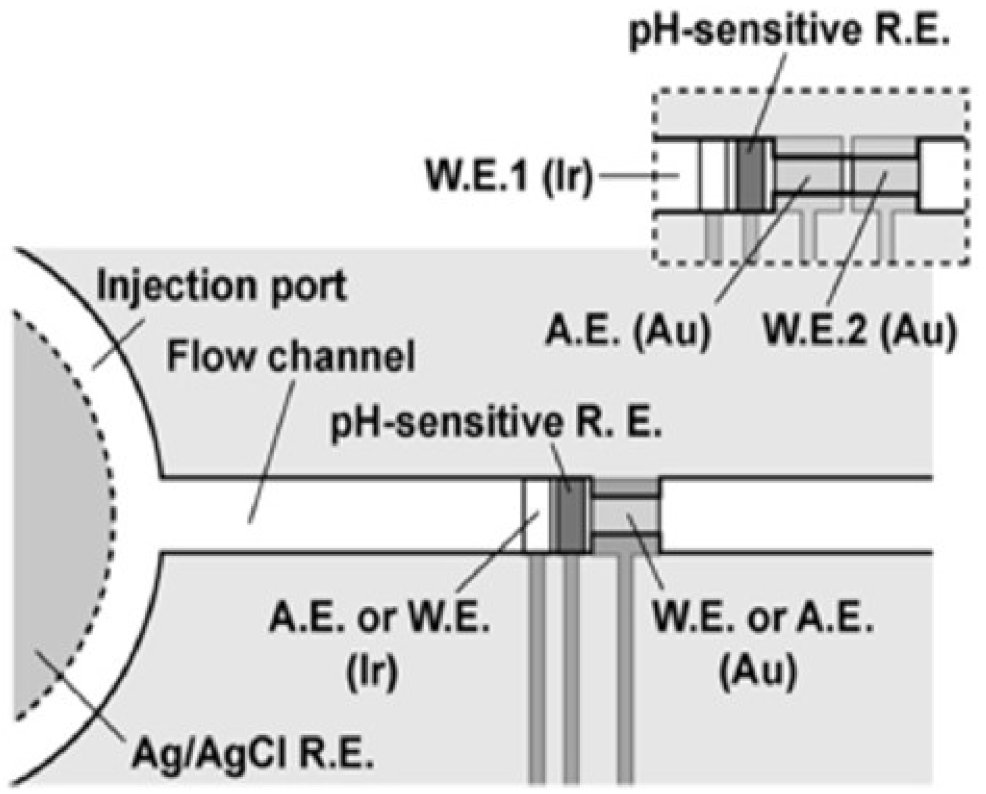
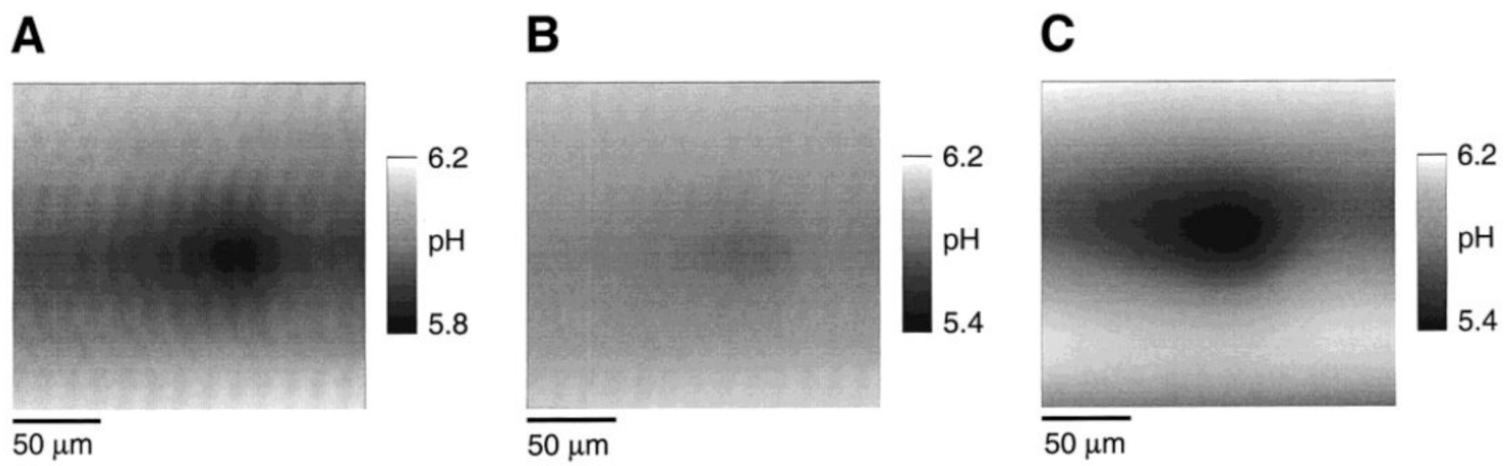
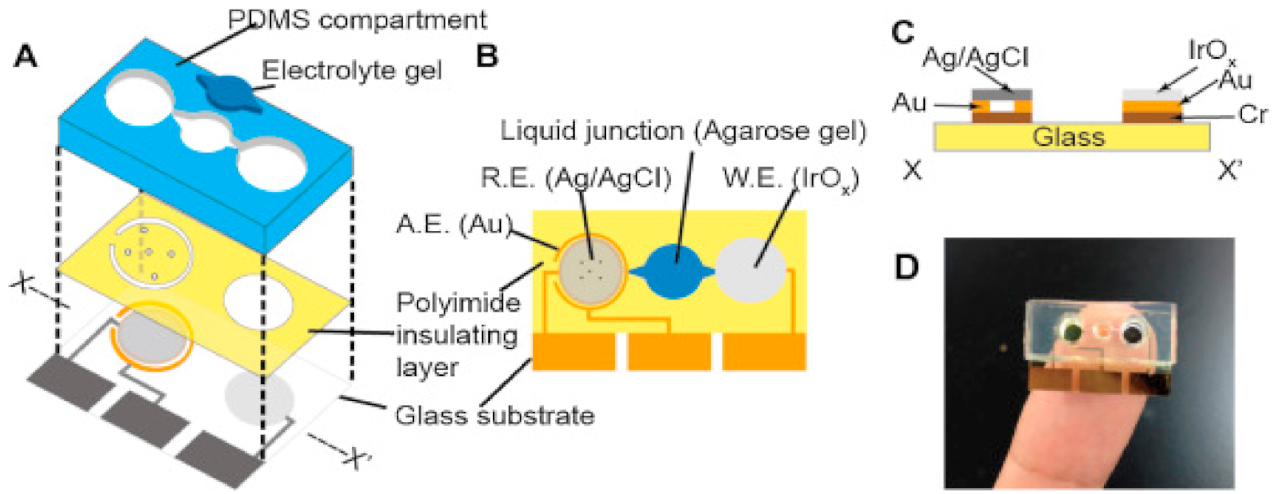
3.3.3. Nanoliter Cell Trap System for Local pH Change Recording
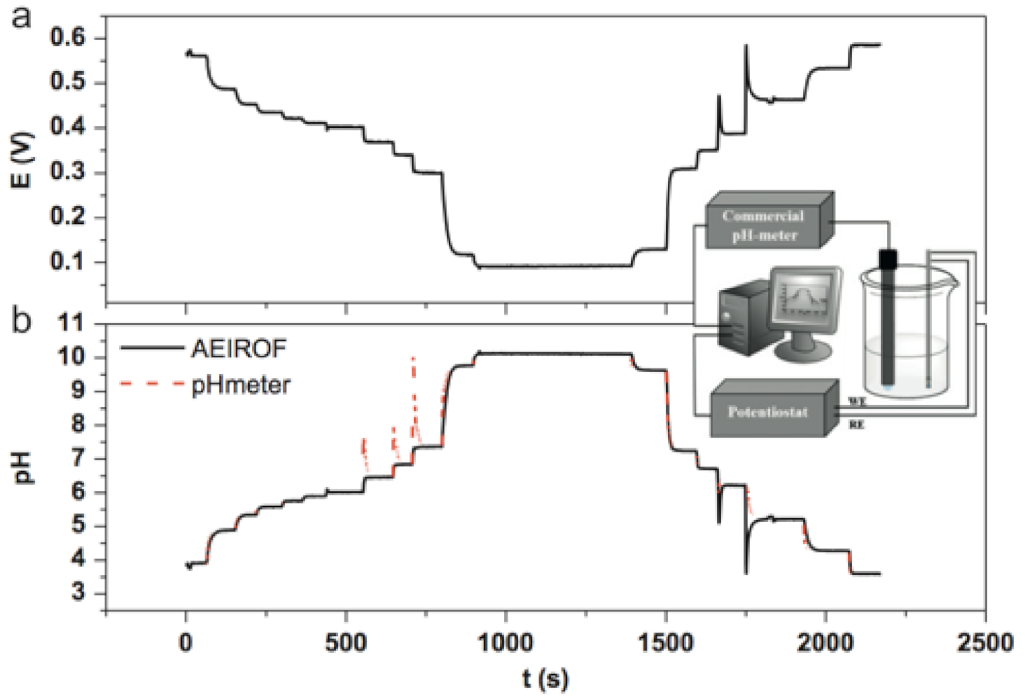
3.3.4. Remote pH Monitoring of Drinking Water
3.3.5. Anodic Aluminum Oxide Template Directed Iridium Oxide for pH Sensing
3.3.6. Microelectrode for pH Sensing
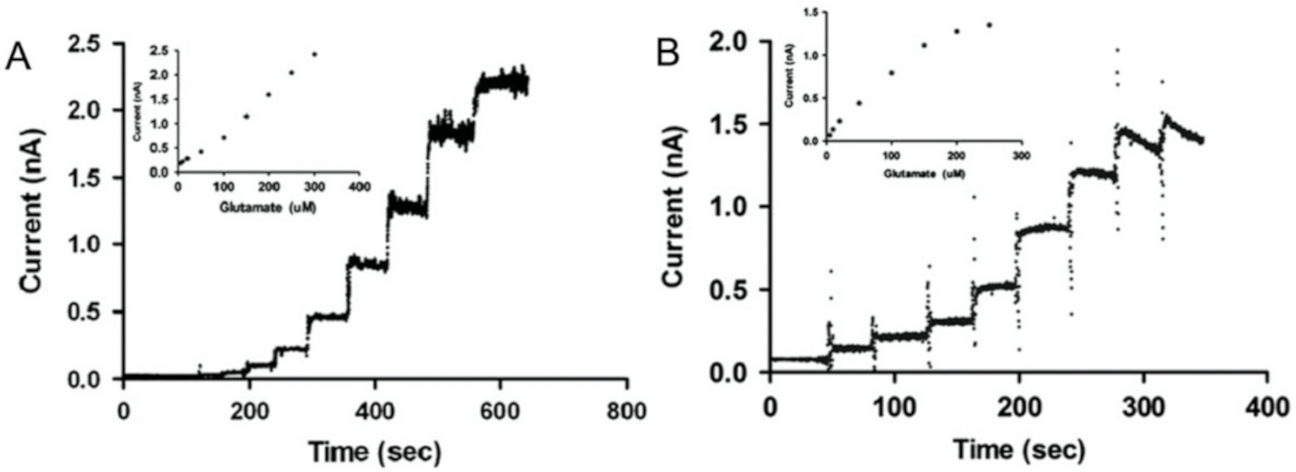
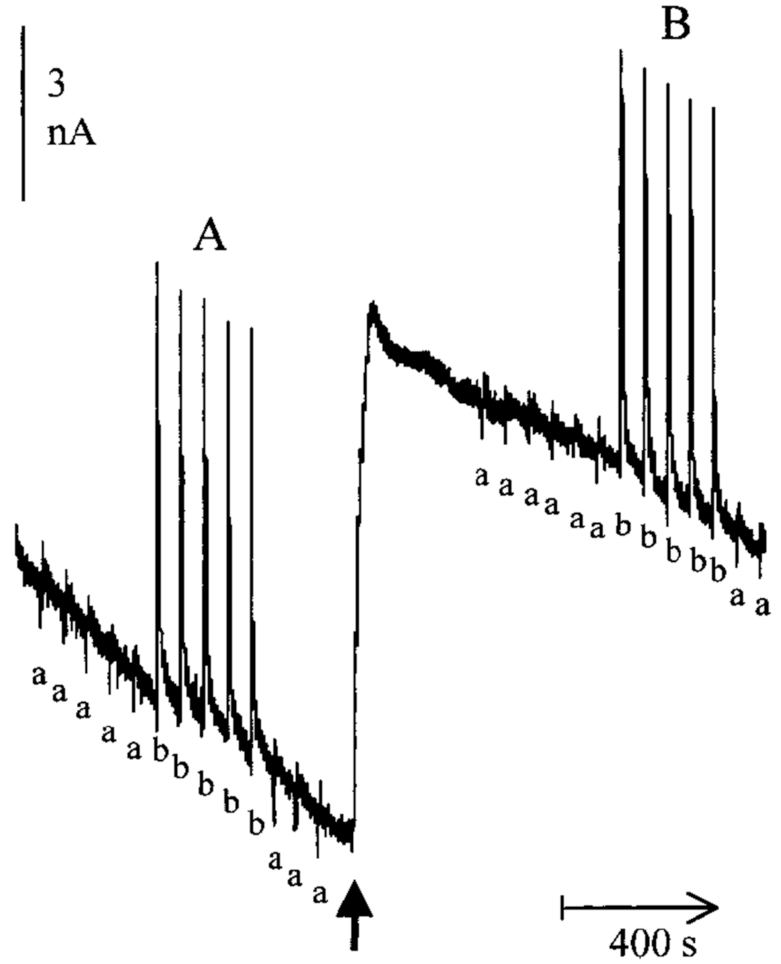
3.4. Glutamate Sensor
3.5. Immunosensor Composed of Iridium Oxides for IgG and Human Transferrin Detection
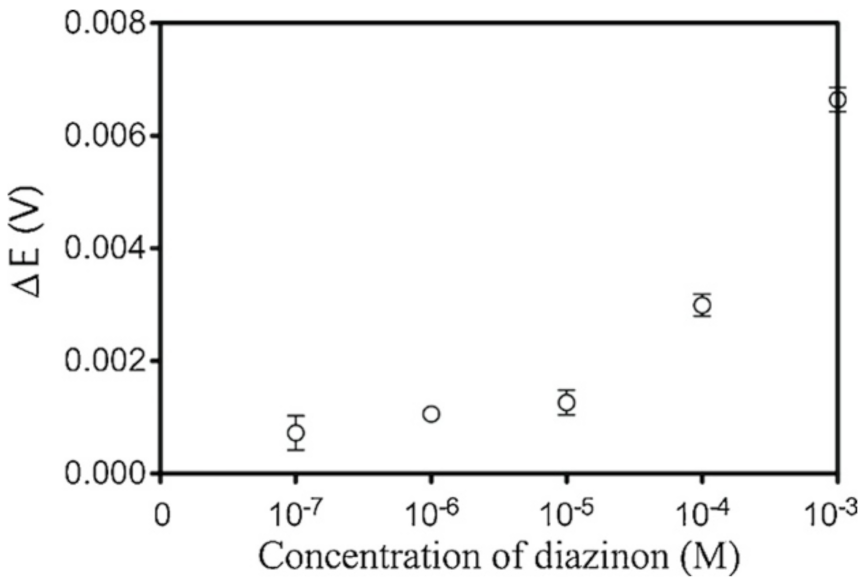
3.6. Organophosphate Pesticides (Ops) Detection
3.7. Metal Ions Detection
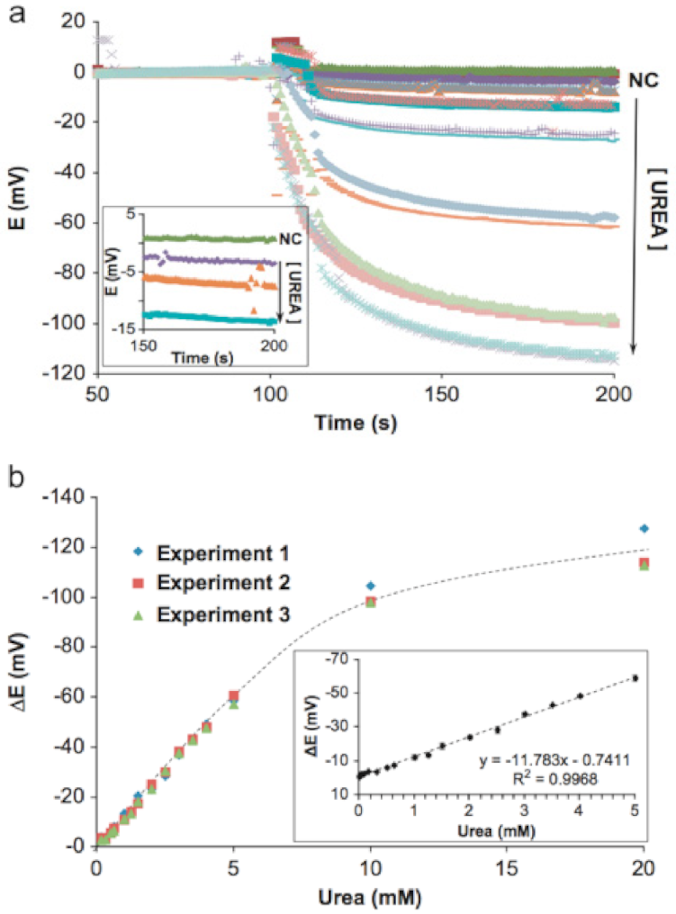
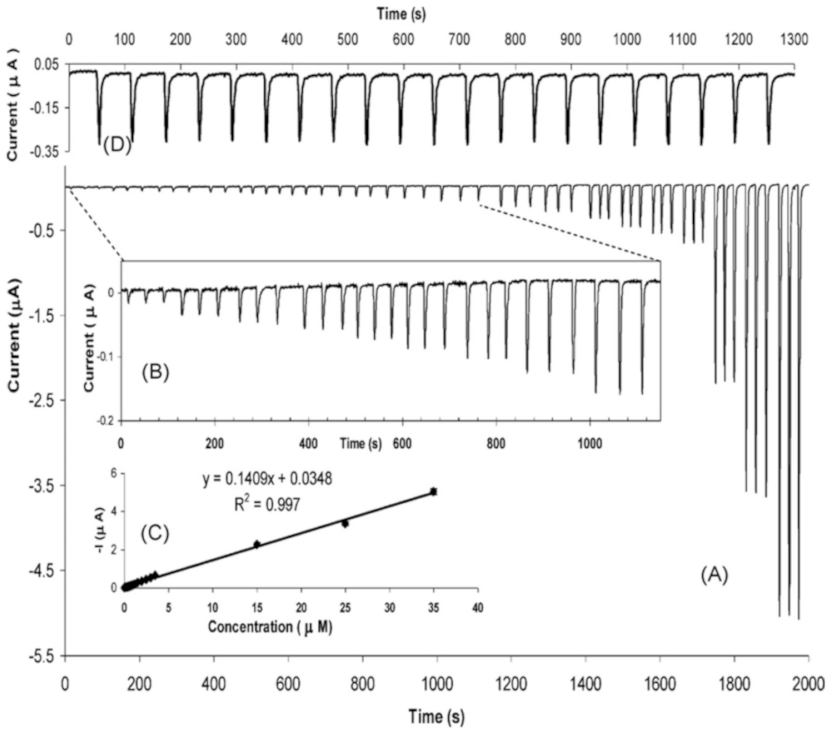
3.8. Urea Detection
3.9. Protein Detection
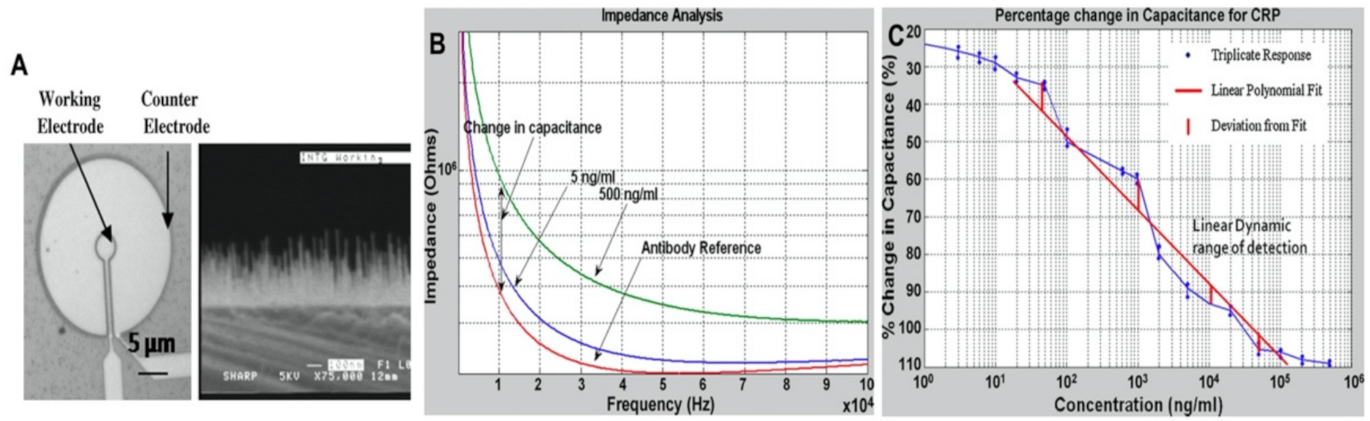
3.9.1. C-Reactive Protein (CRP) and Myeloperoxidase (MPO)
3.9.2. Insulin Detection
3.10. Iodate and Periodate Detection
3.11. Metalloid Ions Detection-Arsenic (III) Ions
3.12. Ester Type Chemical Detection
3.13. Nanotube of Iridium Oxide for Cell Action Potential Recording
3.14. Iridium Oxide-Based Gas Sensors
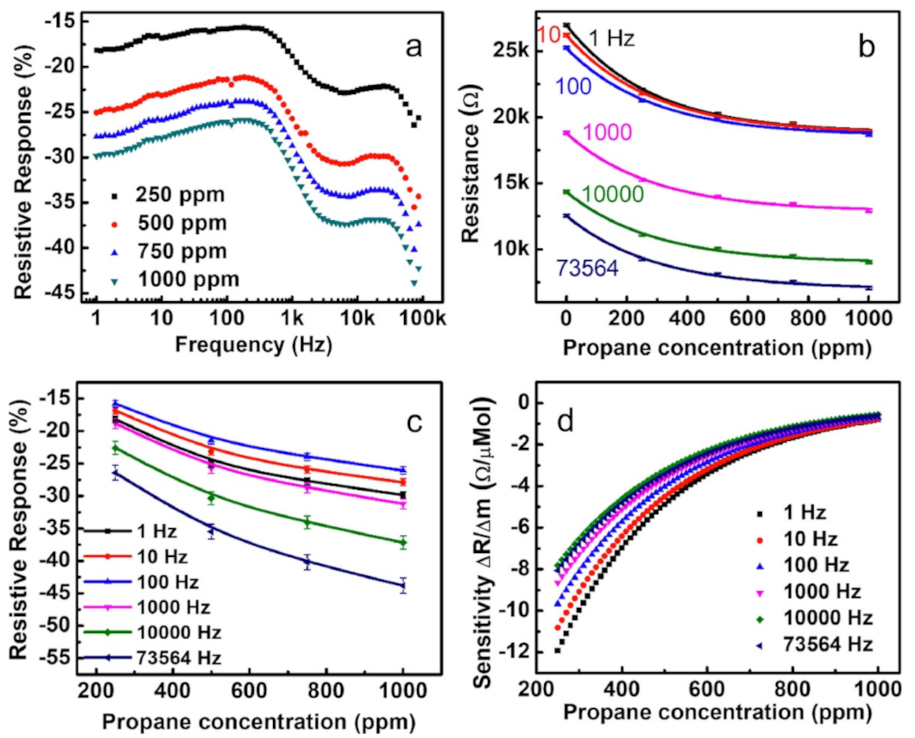
3.14.1. Iridium Oxide-Based Propane Sensors
3.14.2. Iridium Oxide-Based Hazardous Gases Detection
3.14.3. The Multi-Electrode Gas Sensing Configuration for the High-Throughput Impedance Screening
3.15. Iridium Oxides Applications in Monitoring Nucleic Acid Amplification
4. Conclusions and the Future Trends of Iridium Oxides-Based Sensors
4.1. Future Trends and Developments in the Biosensors for Iridium Oxide-Based Materials
4.2. Future Trends and Developments in Gas Sensors for Iridium Oxide-Based Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamanaka, K. Anodically Electrodeposited Iridium Oxide Films (AEIROF) from Alkaline Solutions for Electrochromic Display Devices. Jpn. J. Appl. Phys. 1989, 28, 632–637. [Google Scholar] [CrossRef]
- Terashima, C.; Rao, T.N.; Sarada, B.V.; Spataru, N.; Fujishima, A. Electrodeposition of hydrous iridium oxide on conductive diamond electrodes for catalytic sensor applications. J. Electroanal. Chem. 2003, 544, 65–74. [Google Scholar] [CrossRef]
- Kinoshita, K.; Madou, M. Electrochemical measurements on Pt, Ir, and Ti oxides as pH probes. J. Electrochem. Soc. 1984, 131, 1089–1094. [Google Scholar] [CrossRef]
- Ardizzone, S.; Carugati, A.; Trasatti, S. Properties of thermally prepared iridium dioxide electrodes. J. Electroanal. Chem. Interfac. Electrochem. 1981, 126, 287–292. [Google Scholar] [CrossRef]
- Chalamala, B.R.; Wei, Y.; Reuss, R.H.; Aggarwal, S.; Gnade, B.E.; Ramesh, R.; Bernhard, J.M.; Sosa, E.D.; Golden, D.E. Effect of growth conditions on surface morphology and photoelectric work function characteristics of iridium oxide thin films. Appl. Phys. Lett. 1999, 74, 1394–1396. [Google Scholar] [CrossRef]
- Kinlen, P.J.; Heider, J.E.; Hubbard, D.E. A solid-state pH sensor based on a Nafion-coated iridium oxide indicator electrode and a polymer-based silver chloride reference electrode. Sens. Actuators B Chem. 1994, 22, 13–25. [Google Scholar] [CrossRef]
- Klein, J.D.; Clauson, S.L.; Cogan, S.F. Morphology and charge capacity of sputtered iridium oxide films. J. Vac. Sci. Technol. A 1989, 7, 3043–3047. [Google Scholar] [CrossRef]
- Kreider, K. IrO2 radio frequency sputtered thin film properties. J. Vac. Sci. Technol. A 1986, 4, 606–607. [Google Scholar] [CrossRef]
- Sato, Y. Electrochromis in Thermally Oxidized Iridium Oxide Films in LiClO4/Propylene Carbonate. Jpn. J. Appl. Phys. 1989, 28, 1290–1291. [Google Scholar] [CrossRef]
- Yao, S.; Wang, M.; Madou, M. A pH Electrode Based on Melt-Oxidized Iridium Oxide. J. Electrochem. Soc. 2001, 148, H29–H36. [Google Scholar] [CrossRef]
- Yagi, M.; Tomita, E.; Kuwabara, T. Remarkably high activity of electrodeposited IrO2 film for electrocatalytic water oxidation. J. Electroanal. Chem. 2005, 579, 83–88. [Google Scholar] [CrossRef]
- Jhas, A.S.; Elzanowska, H.; Sebastian, B.; Birss, V. Dual oxygen and Ir oxide regeneration of glucose oxidase in nanostructured thin film glucose sensors. Electrochim. Acta 2010, 55, 7683–7689. [Google Scholar] [CrossRef]
- Campbell, H.; Elzanowska, H.; Birss, V. Towards a reliable and high sensitivity O2-independent glucose sensor based on Ir oxidenanoparticles. Biosens. Bioelectron. 2013, 42, 563–569. [Google Scholar] [CrossRef]
- Kotzian, P.; Brázdilová, P.; Kalcher, K.; Handlíř, K.; Vytřas, K. Oxides of platinum metal group as potential catalysts in car-bonaceous amperometric biosensors based on oxidases. Sens. Actuators B Chem. 2007, 124, 297–302. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Morimoto, K.; Fukuda, J.; Suzuki, H. Electrowetting-based pH- and biomolecule-responsive valves and pH filters. Biosens. Bioelectron. 2009, 24, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Abu Irhayem, E.; Elzanowska, H.; Jhas, A.S.; Skrzynecka, B.; Birss, V. Glucose detection based on electrochemically formed Ir oxide films. J. Electroanal. Chem. 2002, 538-539, 153–164. [Google Scholar] [CrossRef]
- Wipf, D.O.; Ge, F.; Spaine, T.W.; Baur, J.E. Microscopic Measurement of pH with Iridium Oxide Microelectrodes. Anal. Chem. 2000, 72, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- KiláKang, S.; AuckáChoi, C.; ShináKim, Y.; TaeáKim, Y. An iridium oxide reference electrode for use in microfabricated biosensors and biochips. Lab Chip 2004, 4, 42–46. [Google Scholar]
- Marzouk, S.A.; Ufer, S.; Buck, R.P.; Johnson, T.A.; Dunlap, L.A.; Cascio, W.E. Electrodeposited iridium oxide pH electrode for meas-urement of extracellular myocardial acidosis during acute ischemia. Anal. Chem. 1998, 70, 5054–5061. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Hsu, T.-K.; Sun, C.-L.; Nien, Y.-T.; Pu, N.-W.; Ger, M.-D. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Li, C.; Ahn, C.H.; Shutter, L.; Narayan, R.K. Toward real-time continuous brain glucose and oxygen monitoring with a smart catheter. Biosens. Bioelectron. 2009, 25, 173–178. [Google Scholar] [CrossRef]
- Li, C.; Limnuson, K.; Wu, Z.; Amin, A.; Narayan, A.; Golanov, E.; Ahn, C.H.; Hartings, J.; Narayan, R.K. Single probe for real-time simultaneous monitoring of neurochemistry and direct-current electrocorticography. Biosens. Bioelectron. 2016, 77, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Ribet, F.; Stemme, G.; Roxhed, N. Ultra-miniaturization of a planar amperometric sensor targeting continuous intradermal glucose monitoring. Biosens. Bioelectron. 2017, 90, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Dong, Q.; Song, D.; Huang, Y.; Xu, Z.; Chapman, J.H.; Willis, W.S.; Li, B.; Lei, Y. High-temperature annealing enabled iridium oxide nanofibers for both non-enzymatic glucose and solid-state pH sensing. Electrochim. Acta 2018, 281, 117–126. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, X.; Liu, H.; Ryu, H.; Zhao, J.; Li, B.; Lei, Y. Heterogeneous Iridium Oxide/Gold Nanocluster for Non-enzymatic Glucose Sensing and pH Probing. Eng. Sci. 2019, 8, 46–53. [Google Scholar] [CrossRef]
- Morimitsu, M.; Okazaki, A. A Novel Electrode Material for H2O2 Oxidation of Electrochemical Glucose Sensors. ECS Meet. Abstr. 2009, 19, 6. [Google Scholar] [CrossRef]
- Ha, Y.; Jung, H.; Lee, C.; Kim, M.H.; Lee, Y. Alteration of the morphology and electrocatalytic activity of IrO2 nanowires upon reduction by hydrogen gas. Sens. Actuators B Chem. 2015, 216, 159–164. [Google Scholar] [CrossRef]
- Smiechowski, M.F.; Lvovich, V.F. Iridium oxide sensors for acidity and basicity detection in industrial lubricants. Sens. Actuators B Chem. 2003, 96, 261–267. [Google Scholar] [CrossRef]
- Tarlov, M.; Semancik, S.; Kreider, K. Mechanistic and response studies of iridium oxide pH sensors. Sens. Actuators B Chem. 1990, 1, 293–297. [Google Scholar] [CrossRef]
- Kakooei, S.; Ismail, M.C.; Ari-Wahjoedi, B. An overview of pH sensors based on iridium oxide: Fabrication and application. Int. J. Mater Sci. Innovat. 2013, 1, 62–72. [Google Scholar]
- Elsen, H.A.; Monson, C.F.; Majda, M. Effects of Electrodeposition Conditions and Protocol on the Properties of Iridium Oxide pH Sensor Electrodes. J. Electrochem. Soc. 2009, 156, F1–F6. [Google Scholar] [CrossRef] [Green Version]
- Rouhi, J.; Kakooei, S.; Ismail, M.C.; Karimzadeh, R.; Mahmood, M.R. Development of iridium oxide sensor for surface pH meas-urement of a corroding metal under deposit. Int. J. Electrochem. Sci. 2017, 12, 9933–9943. [Google Scholar] [CrossRef]
- Jovic, M.; Hidalgo-Acosta, J.C.; Lesch, A.; Bassetto, V.C.; Smirnov, E.; Cortés-Salazar, F.; Girault, H.H. Large-scale layer-by-layer inkjet printing of flexible iridium-oxide based pH sensors. J. Electroanal. Chem. 2018, 819, 384–390. [Google Scholar] [CrossRef]
- Izutsu, K.; Yamamoto, H. Response of an iridium oxide pH-sensor in nonaqueous solutions. Comparison with other pH-sensors. Anal. Sci. 1996, 12, 905–909. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Liu, X.; Ye, Z.; Zhang, J.; Cao, F.; Zhang, J. A fabrication of iridium oxide film pH micro-sensor on Pt ultramicroelectrode and its application on in-situ pH distribution of 316L stainless steel corrosion at open circuit potential. Sens. Actuators B Chem. 2018, 255, 1974–1982. [Google Scholar] [CrossRef]
- Hitchman, M.L.; Ramanathan, S. Evaluation of iridium oxide electrodes formed by potential cycling as pH probes. Analyst 1988, 113, 35–39. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Rao, S.; Seo, Y.-S.; Schadt, K.; Hao, Y.; Chiao, J.-C. Micro pH Sensors Based on Iridium Oxide Nanotubes. IEEE Trans. Nanotechnol. 2014, 13, 945–953. [Google Scholar] [CrossRef]
- Yang, J.; Kwak, T.J.; Zhang, X.; McClain, R.; Chang, W.-J.; Gunasekaran, S. Digital pH Test Strips for In-Field pH Monitoring Using Iridium Oxide-Reduced Graphene Oxide Hybrid Thin Films. ACS Sens. 2016, 1, 1235–1243. [Google Scholar] [CrossRef]
- Khalil, M.; Wang, S.; Yu, J.; Lee, R.L.; Liu, N. Electrodeposition of Iridium Oxide Nanoparticles for pH Sensing Electrodes. J. Electrochem. Soc. 2016, 163, B485–B490. [Google Scholar] [CrossRef]
- Huang, X.-R.; Ren, Q.-Q.; Yuan, X.-J.; Wen, W.; Chen, W.; Zhan, D.-P. Iridium oxide based coaxial pH ultramicroelectrode. Electro Chem. Commun. 2014, 40, 35–37. [Google Scholar] [CrossRef]
- Madeira, G.D.M.; Mello, H.J.N.P.D.; Faleiros, M.C.; Mulato, M. Model improvement for super-Nernstian pH sensors: The effect of surface hydration. J. Mater. Sci. 2020, 56, 2738–2747. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655. [Google Scholar] [CrossRef]
- Ges, I.; Baudenbacher, F. Microfluidic device to confine single cardiac myocytes in sub-nanoliter volumes for extracellular pH measurements. J. Exp. Nanosci. 2008, 3, 63–75. [Google Scholar] [CrossRef]
- Ges, I.A.; Ivanov, B.L.; Werdich, A.A.; Baudenbacher, F.J. Differential pH measurements of metabolic cellular activity in nl culture volumes using microfabricated iridium oxide electrodes. Biosens. Bioelectron. 2007, 22, 1303–1310. [Google Scholar] [CrossRef]
- Ges, I.A.; Ivanov, B.L.; Schaffer, D.K.; Lima, E.A.; Werdich, A.A.; Baudenbacher, F.J. Thin-film IrO x pH microelectrode for microflu-idic-based microsystems. Biosens. Bioelectron. 2005, 21, 248–256. [Google Scholar] [CrossRef]
- Carroll, S.; Baldwin, R.P. Self-Calibrating Microfabricated Iridium Oxide pH Electrode Array for Remote Monitoring. Anal. Chem. 2010, 82, 878–885. [Google Scholar] [CrossRef]
- Lin, Z.C.; Xie, C.; Osakada, Y.; Cui, Y.; Cui, B. Iridium oxide nanotube electrodes for sensitive and prolonged intracellular meas-urement of action potentials. Nat. Commun. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.M.; Gurung, I.; Cao, H.; Rao, S.; Chiao, J.-C. Fabrication of pH-sensing iridium oxide nanotubes on patterned electrodes using anodic aluminum oxide nanotemplate. In Proceedings of the SENSORS, 2013 IEEE, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Comstock, D.J.; Christensen, S.T.; Elam, J.W.; Pellin, M.J.; Hersam, M.C. Synthesis of nanoporous activated iridium oxide films by anodized aluminum oxide templated atomic layer deposition. Electrochem. Commun. 2010, 12, 1543–1546. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, T.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sens. Actuators B Chem. 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, J.; Tak, Y. Electrochromic mechanism of IrO2 prepared by pulsed anodic electrodeposition. Electrochem. Solid State Lett. 2004, 7, H5–H8. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Rao, S.; Yang, X.; Dubey, S.; Mays, J.; Cao, H.; Chiao, J.-C. Sol-Gel Deposition of Iridium Oxide for Biomedical Micro-Devices. Sensors 2015, 15, 4212–4228. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yokokawa, M.; Satake, T.; Suzuki, H. A micro IrO x potentiometric sensor for direct determination of organophosphate pesticides. Sens. Actuators B Chem. 2015, 220, 859–863. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Zhang, T.C. Fabrication of anodically electrodeposited iridium oxide film pH microelectrodes for microenvi-ronmental studies. Anal. Chem. 2002, 74, 5726–5733. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, Y.; Kim, J.; Gu, J.; Wang, J.; Park, I. Biopsy needle integrated with multi-modal physical/chemical sensor array. Biosens. Bioelectron. 2019, 148, 111822. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.K.; Johnson, M.D.; Scottt, K.A.; Shim, J.H.; Nam, H.; Kipket, D.R.; Brown, R.B. Iridium oxide reference electrodes for neu-rochemical sensing with MEMS microelectrode arrays. In Proceedings of the SENSORS, 2005 IEEE, Irvine, CA, USA, 30 October–3 November 2005. [Google Scholar]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D. A Low-Cost pH Sensor Based on RuO2 Resistor Material. Nano Hybrids 2013, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, G.; Lemos, S.; Pocrifka, L.; Marreto, P.; Rosario, A.; Pereira, E. Development of low-cost metal oxide pH electrodes based on the polymeric precursor method. Anal. Chim. Acta 2008, 616, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Mazzaracchio, V.; Fiore, L.; Nappi, S.; Marrocco, G.; Arduini, F. Medium-distance affordable, flexible and wireless epidermal sensor for pH monitoring in sweat. Talanta 2020, 222, 121502. [Google Scholar] [CrossRef]
- Hendrikse, J.; Olthuis, W.; Bergveld, P. A drift free nernstian iridium oxide pH sensor. In Proceedings of the International Solid State Sensors and Actuators Conference (Transducers’ 97), Chicago, IL, USA, 19 June 1997. [Google Scholar]
- Wang, M.; Yao, S.; Madou, M. A long-term stable iridium oxide pH electrode. Sens. Actuators B Chem. 2002, 81, 313–315. [Google Scholar] [CrossRef]
- Ratanaporncharoen, C.; Tabata, M.; Watanagool, N.; Goda, T.; Matsumoto, A.; Sriyudthsak, M.; Miyahara, Y. Characterization and opti-mization of thermally grown iridium oxide and its application to pH sensors. Sens. Mater. 2018, 30, 1175–1185. [Google Scholar]
- Mingels, R.; Kalsi, S.; Cheong, Y.; Morgan, H. Iridium and Ruthenium oxide miniature pH sensors: Long-term performance. Sens. Actuators B Chem. 2019, 297, 126779. [Google Scholar] [CrossRef]
- Marsh, P.; Manjakkal, L.; Yang, X.; Huerta, M.; Le, T.; Thiel, L.; Chiao, J.C.; Cao, H.; Dahiya, R. Flexible iridium oxide based pH sensor integrated with inductively coupled wireless transmission system for wearable applications. IEEE Sens. J. 2020, 20, 5130–5138. [Google Scholar] [CrossRef] [Green Version]
- Tolosa, V.M.; Wassum, K.M.; Maidment, N.T.; Monbouquette, H.G. Electrochemically deposited iridium oxide reference electrode integrated with an electroenzymatic glutamate sensor on a multi-electrode arraymicroprobe. Biosens. Bioelectron. 2013, 42, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Rauh, R. Novel amperometric immunosensors based on iridium oxide matrices. Biosens. Bioelectron. 2004, 19, 693–699. [Google Scholar] [CrossRef]
- Badawy, M.E.; El-Aswad, A.F. Bioactive paper sensor based on the acetylcholinesterase for the rapid detection of organophos-phate and carbamate pesticides. Int. J. Anal. Chem. 2014, 2014, 536823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capoferri, D.; Álvarez-Diduk, R.; Del Carlo, M.; Compagnone, D.; Merkoçi, A. Electrochromic molecular imprinting sensor for visual and smartphone-based detections. Anal. Chem. 2018, 90, 5850–5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- vel Krawczyk, T.K.; Moszczyńska, M.; Trojanowicz, M. Inhibitive determination of mercury and other metal ions by potenti-ometric urea biosensor. Biosens. Bioelectron. 2000, 15, 681–691. [Google Scholar] [CrossRef]
- Greenan, N.; Mulvaney, R.; Sims, G.K. A microscale method for colorimetric determination of urea in soil extracts. Commun. Soil Sci. Plant Anal. 1995, 26, 2519–2529. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, M.; Flöck, M.; Winter, P.; Al, E. Evaluation of flow injection analysis for determination of urea in sheep’s and cow’s milk. Acta Vet. Hung. 2002, 50, 263–271. [Google Scholar] [CrossRef]
- Francis, P.; Lewis, S.W.; Lim, K. Analytical methodology for the determination of urea: Current practice and future trends. TrAC Trends Anal. Chem. 2002, 21, 389–400. [Google Scholar] [CrossRef]
- Alfonso, E.P.; Abad, L.; Casan-Pastor, N.; Gonzalo-Ruiz, J.; Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea–urease in real urine samples. Biosens. Bioelectron. 2013, 39, 163–169. [Google Scholar] [CrossRef]
- Venkatraman, V.L.; Reddy, R.K.; Zhang, F.; Evans, D.; Ulrich, B.; Prasad, S. Iridium oxide nanomonitors: Clinical diagnostic devices for health monitoring systems. Biosens. Bioelectron. 2009, 24, 3078–3083. [Google Scholar] [CrossRef]
- Zhang, F.; Ulrich, B.; Reddy, R.K.; Venkatraman, V.L.; Prasad, S.; Vu, T.Q.; Hsu, S.-T. Fabrication of Submicron IrO2Nanowire Array Biosensor Platform by Conventional Complementary Metal–Oxide–Semiconductor Process. Jpn. J. Appl. Phys. 2008, 47, 1147–1151. [Google Scholar] [CrossRef]
- Pikulski, M.; Gorski, W. Iridium-based electrocatalytic systems for the determination of insulin. Anal. Chem. 2000, 72, 2696–2702. [Google Scholar] [CrossRef]
- Salimi, A.; Hallaj, R.; Kavosi, B.; Hagighi, B. Highly sensitive and selective amperometric sensors for nanomolar detection of iodate and periodate based on glassy carbon electrode modified with iridium oxide nanoparticles. Anal. Chim. Acta 2010, 661, 28–34. [Google Scholar] [CrossRef]
- Salimi, A.; Hyde, M.E.; Banks, C.; Compton, R.G. Boron doped diamond electrode modified with iridium oxide for amperometic detection of ultra trace amounts of arsenic(iii). Analyst 2003, 129, 9–14. [Google Scholar] [CrossRef]
- Roberts, D.C.; Osborn, J.A.; Yacynych, A.M. Proteolytic enzyme modified metal oxide electrodes as potentiometric sensors. Anal. Chem. 1986, 58, 140–144. [Google Scholar] [CrossRef]
- Avila-García, A.; Chaudhary, A.; Rojas-Chávez, H. Iridium oxide films as propane sensors. Thin Solid Films 2021, 724, 138617. [Google Scholar] [CrossRef]
- Hullavarad, N.V.; Hullavarad, S.S. Direct-Vapor-Phase Grown IrO2 Micronanostructures for Ethanol, Acetone, and Propanol Gas Sensor. IEEE Trans. Nanotechnol. 2010, 9, 625–629. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Zhang, C.; Zhang, Q.; Li, Z.; Zhang, S. A high throughput platform screening of ppb-level sensitive materials for hazardous gases. Sens. Actuators B Chem. 2018, 276, 189–203. [Google Scholar] [CrossRef]
- Navarrete, E.; Bittencourt, C.; Umek, P.; Cossement, D.; Güell, F.; Llobet, E. Tungsten trioxide nanowires decorated with iridium oxide nanoparticles as gas sensing material. J. Alloy. Compd. 2019, 812, 152156. [Google Scholar] [CrossRef]
- Casanova-Cháfer, J.; Navarrete, E.; Noirfalise, X.; Umek, P.; Bittencourt, C.; Llobet, E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors 2018, 19, 113. [Google Scholar] [CrossRef] [Green Version]
- Koplin, T.J.; Siemons, M.; Océn-Valéntin, C.; Sanders, D.; Simon, U. Workflow for high throughput screening of gas sensing mate-rials. Sensors 2006, 6, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, K.; Patterson, A.S.; Ferguson, B.S.; Plaxco, K.W.; Soh, H.T. Rapid, Sensitive, and Quantitative Detection of Pathogenic DNA at the Point of Care through Microfluidic Electrochemical Quantitative Loop-Mediated Isothermal Amplification. Angew. Chem. 2012, 124, 4980–4984. [Google Scholar] [CrossRef]
- Zhu, Z.; Lei, J.; Liu, L.; Ju, H. Label-free electrochemical DNA sensing with a one-target-multitriggered hybridization chain reac-tion strategy. Analyst 2013, 138, 5995–6000. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Su, J.; Xiang, Y.; Yuan, R.; Chai, Y. In situ hybridization chain reaction amplification for universal and highly sen-sitive electrochemiluminescent detection of DNA. Anal. Chem. 2012, 84, 7750–7755. [Google Scholar] [CrossRef]
- Xuan, F.; Luo, X.; Hsing, I.-M. Sensitive immobilization-free electrochemical DNA sensor based on isothermal circular strand displacement polymerization reaction. Biosens. Bioelectron. 2012, 35, 230–234. [Google Scholar] [CrossRef]
- Deféver, T.; Druet, M.; Rochelet-Dequaire, M.; Joannes, M.; Grossiord, C.; Limoges, B.; Marchal, D. Real-Time Electrochemical Monitoring of the Polymerase Chain Reaction by Mediated Redox Catalysis. J. Am. Chem. Soc. 2009, 131, 11433–11441. [Google Scholar] [CrossRef] [PubMed]
- Kivlehan, F.; Mavré, F.; Talini, L.; Limoges, B.; Marchal, D. Real-time electrochemical monitoring of isothermal helicase-dependent amplification of nucleic acids. Analyst 2011, 136, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, M.; Tang, D.; Gao, Z.; Tang, J.; Chen, G. Nanogold-based bio-bar codes for label-free immunosensing of proteins cou-pling with an in situ DNA-based hybridization chain reaction. Chem. Commun. 2012, 48, 12207–12209. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef]
- Tabata, M.; Katayama, Y.; Mannan, F.; Seichi, A.; Suzuki, K.; Goda, T.; Matsumoto, A.; Miyahara, Y. Label-free and Electrochemical Detection of Nucleic Acids Based on Isothermal Amplification in Combination with Solid-state pH Sensor. Procedia Eng. 2016, 168, 419–422. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Chamorro-García, A.; Serrano, L.; Rivas, L.; Quesada-Gonzalez, D.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. An iridium oxide na-noparticle and polythionine thin film based platform for sensitive Leishmania DNA detection. J. Mater. Chem. B 2015, 3, 5166–5171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhou, Y.; Li, Y.; Gu, W.; Zhang, Q.; Liu, J. Luminescent Iridium(III) Complex Labeled DNA for Graphene Oxide-Based Biosensors. Anal. Chem. 2016, 88, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Yang, H.; Mannan, F.; Katayama, Y.; Goda, T.; Matsumoto, A.; Seichi, A.; Suzuki, K.; Miyahara, Y. Electrochemical real-time monitoring of isothermal nucleic acid amplification for quantitative analysis. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1553–1556. [Google Scholar] [CrossRef]
- Salm, E.; Zhong, Y.; Reddy, B., Jr.; Duarte-Guevara, C.; Swaminathan, V.; Liu, Y.S.; Bashir, R. Electrical detection of nucleic acid ampli-fication using an on-chip quasi-reference electrode and a PVC REFET. Anal. Chem. 2014, 86, 6968–6975. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yin, K.; Ding, X.; Li, Z.; Sun, X.; Li, B.; Lalla, R.V.; Gross, R.; Liu, C. An integrated E-Tube cap for sample preparation, isothermal amplification and label-free electrochemical detection of DNA. Biosens. Bioelectron. 2021, 186, 113306. [Google Scholar] [CrossRef]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.J.A.; Ou, C.P.; Amin-Desai, K.; Athanasiou, P.; et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Lee, Y.-J.; Lee, H.-J.; Hong, Y.; Yook, J.-G.; Chung, M.H.; Cho, W.; Choi, H.H. A gas sensor using double split-ring resonator coated with conducting polymer at microwave frequncies. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 1815–1818. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Le, N.-B. Coupling coefficient determination based on simulation and experiment for ST-Cut quartz saw delay-line response. Microsyst. Technol. 2007, 14, 615–622. [Google Scholar] [CrossRef]
- Zakaria, M.; Hashim, U.; Amin, M.M.; Adam, T.; Al-Mufti, M.W. Simulation of 1Ghz center frequency saw using cst software for biosensor application. Middle-East J. Sci. Res. 2013, 18, 1286–1291. [Google Scholar]
- Yuan, H.; Tao, J.; Li, N.; Karmakar, A.; Tang, C.; Cai, H.; Pennycook, S.J.; Singh, N.; Zhao, D. On-Chip Tailorability of Capacitive Gas Sensors Integrated with Metal–Organic Framework Films. Angew. Chem. Int. Ed. 2019, 58, 14089–14094. [Google Scholar] [CrossRef]
- Homayoonnia, S.; Zeinali, S. Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sens. Actuators B Chem. 2016, 237, 776–786. [Google Scholar] [CrossRef]
- Chidambaram, A.; Stylianou, K.C. Electronic metal–organic framework sensors. Inorg. Chem. Front. 2018, 5, 979–998. [Google Scholar] [CrossRef]
- Pham, T.; Li, G.; Bekyarova, E.; Itkis, M.E.; Mulchandani, A. MoS2-Based Optoelectronic Gas Sensor with Sub-parts-per-billion Limit of NO2 Gas Detection. ACS Nano 2019, 13, 3196–3205. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Sun, X.; He, S. Iridium Oxide Enabled Sensors Applications. Catalysts 2021, 11, 1164. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101164
Dong Q, Sun X, He S. Iridium Oxide Enabled Sensors Applications. Catalysts. 2021; 11(10):1164. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101164
Chicago/Turabian StyleDong, Qiuchen, Xiangcheng Sun, and Songbing He. 2021. "Iridium Oxide Enabled Sensors Applications" Catalysts 11, no. 10: 1164. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101164





