1. Introduction
Titanium dioxide (TiO
2) is considered a suitable compound for decomposing wastes and antimicrobial action due to its photocatalytic nature and because it is a chemically stable, non-toxic, inexpensive, and quite safe substance. In particular, various types of photocatalysts have been developed using TiO
2 for wastewater treatment employing various methods including immobilized catalyst systems, membrane separation, and gravitational separation systems. As the TiO
2 semiconductor band gap energy (3.2 eV) limits its activity when used in visible light, in many of these studies, an important issue to be considered is the activation of the photocatalysts with solar radiation, as this can improve the energy efficiency and consequently the economic viability of the process. In that respect, TiO
2 is doped with various elements to enhance visible light activity. As a result, numerous photocatalysts have been developed, such as composite, co-doped, and co-catalysts compounds, their photocatalytic performance depending strongly on their morphological, structural, and textural properties [
1,
2,
3,
4,
5,
6,
7,
8].
There are many studies regarding the antifungal and antibacterial properties of TiO
2 nanoparticles in various forms against a broad range of both Gram-positive and Gram-negative bacteria, properties needed in sectors such as food, textiles, medicine, water disinfection, and food packaging [
9,
10]. In general, the antimicrobial activity of nanostructured TiO
2 is greatly dependent on the photocatalytic performance of TiO
2, which depends strongly on its morphological, structural, and textural properties, as was mentioned before [
1,
2,
3,
4].
Studies have shown that the crystalline structure and morphology of TiO
2 nanoparticles, are influenced by growth process parameters such as temperatures, starting concentration of precursors, pH, etc. The potential health impact and toxicity to the environment of nanomaterials is also currently an important matter to be addressed. As an example, metal oxide nanoparticles (NPs) conventionally synthesized using chemical methods, have shown different levels of toxicity to test organisms [
11,
12,
13]. Their toxicity seems to be mainly related to the small size that permits easy penetration through cellular membranes and its light dependent properties. One way to avoid nanoparticulate free circulation is to immobilize them onto substrates or larger structures [
14].
Regarding the improvement of the electron–hole pair generation and the enlargement of the spectral absorption domain of TiO
2, many studies have been performed related to the inclusion of metal/non-metal ions in the structure of TiO
2, the dye functionalization on the TiO
2 surface in dye-sensitized solar cells (DSSC), and the growth of noble metals onto the TiO
2 surface [
15]. Silver (Ag) is among the most interesting metals used as a dopant to modify the structure of TiO
2 because it has the particular property to prevent the recombination of electron–hole pairs. Moreover, Ag can generate surface plasmon resonance with TiO
2 under visible light. These changes provided by Ag doping lead to a significant improvement of the photocatalytic activity, a fact confirmed by other authors [
16,
17,
18]. On the other hand, Ag nanoparticles possess a broad spectrum of antibacterial, antifungal, and antiviral properties. Ag nanoparticles have the ability to penetrate bacterial cell walls, changing the structure of cell membranes and even resulting in cell death. Their efficacy is due not only to their nanoscale size but also to their large ratio of surface area to volume. They can increase the permeability of cell membranes, produce reactive oxygen species, and interrupt the replication of deoxyribonucleic acid by releasing silver ions.
There are studies showing that the inclusion of Ag in the structure of TiO
2 leads to enhance photocatalytic efficiency, as well as antimicrobial properties. Over the years, a large volume of reported research was focused on obtaining 0D systems (nanoparticles) based on Ag doped TiO
2, which were tested in terms of photocatalytic and antimicrobial performances [
13,
19,
20,
21,
22]. As an example, studies showed that TiO
2-NPs had efficient antimicrobial activity against
E. coli,
S. aureus, methicillin-resistant
S. aureus,
K. pneumoniae [
23,
24]. However, little attention was paid to the development of Ag doped TiO
2 nanofibers by the electrospinning-calcination technique, as well as studies of their performances in photocatalytic dye degradation and antimicrobial action. As an example, Zhang et al. [
15] prepared hierarchical structures composed of TiO
2 fibers on which Ag nanoparticles were grown to improve the photocatalytic efficiency for Rhodamine B (RhB) dye degradation. Moreover, nano-Ag-decorated TiO
2-nanofibres proved that the inclusion of Ag exhibited an increased antimicrobial effect on
S. aureus and
E. coli [
25]. Recently, Roongraung et al. [
18] reported the photocatalytic performance of Ag doped TiO
2 nanofibers for photocatalytic glucose conversion.
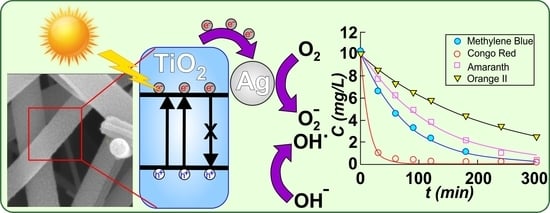
Although the research on TiO2 has a very long history and its applications are almost countless as the respective publications are too, this semiconductor has the potential to offer even today very interesting results worth being further investigated. This paper reports the development and optimization of pure TiO2 and Ag–TiO2 photocatalytic nanostructured nanofibers, fabricated by electrospinning followed by calcination at high temperature. Nanofibers can be well stacked together in larger 3D structures, thus, they can better be immobilized in membranes or other kinds of support, offering at the same time a much larger active surface than that for the case of nano-powders, while they can easily be recovered from the active medium used in applications. At the same time, the presence of Ag can lead to both an effective photocatalytic activity and antimicrobial action and a sunlight-driven activation, as compared to pure TiO2. Therefore, the integration of such materials into food packaging, medical textiles, and other healthcare related items can be a quite innovative approach for real-life applications. In the present study, we report the fabrication of materials with remarkable photocatalytic efficiency of 99% at a constant rate of k = 1.29 × 10−2 min−1 that was found for the 0.1% Ag–TiO2 sample for the case of Methylene Blue dye degradation. Additionally, these materials proved able to degrade up 99% of other organic dyes with constant rates in the range of 4.57 × 10−3 to 7.28 × 10−2 min−1 depending on the dye nature, all degradation tests being performed under UV-visible light irradiation from a halogen lamp, without intervening to acidify the solution or add H2O2. These materials proved to be also reusable.
3. Materials and Methods
3.1. Materials
Titanium (IV) isopropoxide (TTIP), silver nitrate (AgNO3), glacial acetic acid (CH3COOH), ethanol, polyvinylpyrrolidone (PVP) (Mw = 1.300.000) of analytical grade purchased from Sigma-Aldrich (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany) were used for the preparation of the Ag-doped TiO2 nanostructures. Methylene blue, Congo red, orange II, and amaranth dyes were procured from Sigma-Aldrich (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany) and utilized without further purification.
3.2. Preparation of Ag–TiO2 Nanostructured Nanofibers
Pure TiO
2 and Ag–TiO
2 nanostructured nanofibers were developed using electrospun solutions, which were obtained by mixing two initial solutions, solution 1 containing 0.75 mL of TTIP in 1.5 mL acetic acid and solution 2 consisting of 0.25 g PVP in 2.5 mL ethanol. This solution was used to prepare the pure TiO
2 blank sample (named T400), while in the case of Ag–TiO
2 nanostructures, these were prepared by adding to solution 2 different amounts of AgNO
3 as follows: 0.5 mg corresponding to a 0.1% mass percentage (sample TAg1), 2.5 mg for 0.5% (sample TAg2), 5 mg for 1.0% (sample TAg3), 15 mg for 3.0% (sample TAg4) and 25 mg for 5.0% (sample TAg5). The electrospinning was performed using the set-up already described in our previous publications [
47,
48]. The electrospinning parameters used to obtain the fibrous materials were: 25 kV high voltage, 15 cm tip to collector distance, and 0.75 mL/h feed flow rate. The Ag–TiO
2 nanostructured nanofibers were obtained after removal of PVP matrix using calcination in air at 400 °C with a heating rate of 15 °C/min for 4 h.
3.3. Characterization
The crystallographic structure of all materials was studied using X-ray diffraction (XRD) technique performed with a Rigaku SmartLab-9kW diffractometer (Rigaku Corporation, Tokyo, Japan). The experimental spectra were analyzed using PDXL software developed by Rigaku Corp., Tokyo, Japan. The respective morphological characteristics were examined using a Verios G4 UC Scanning Electron Microscope (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an energy dispersive spectrometer (EDX) (AMETEK, Tokyo, Japan), EDAX Octane Elite. The morphology of the fibers based on pure and doped TiO2 was studied using a Hitachi High-Tech HT7700 Transmission Electron Microscope (TEM) (Hitachi, Tokyo, Japan), operated in high contrast mode at 120 kV accelerating voltage. BET analysis was performed using a fully automated gravimetric analyzer IGASorp supplied by Hidden Analytical, Warrington (UK), with an ultrasensitive microbalance, which was used to measure dynamic water vapor sorption capacity of the samples by the weight change with variation of humidity at a constant temperature. Each sample was dried in flowing nitrogen (250 mL/min) until the weight of the sample was in equilibrium at RH < 1%. Experiments were carried out at 25 °C in the relative humidity (RH) range 0–90%, with 10% humidity steps, each having a pre-established equilibrium time between 10 and 20 min (minimum time and time out, respectively).
A Horiba Scientific LabRam HR Evolution spectrometer (HORIBA Scientific, Palaiseau, France) was used for Raman spectroscopy measurements, which were done in the 25–1650 cm−1 frequency range, employing excitation at 532 nm, acquisition time 100 s, 3 accumulations and a grating of 600 gr/mm. The optical properties were evaluated by UV-vis diffuse reflectance spectra, recorded on an Analytik Jena (Jena, Germany) SPECORD UV/Vis 210+ spectrophotometer with an integrating sphere. Emission spectra were recorded employing a luminescence spectrometer Perkin Elmer LS55 (PerkinElmer, Inc., Waltham, MA, USA).
3.4. Photocatalytic Activity
The photocatalytic activity of Ag–TiO
2 nanostructured nanofibers was evaluated by studying the photodegradation of methylene blue (MB), amaranth, Congo red (CR) and orange II dye solutions under UV-visible light irradiation supplied by a 400 W halogen lamp (Model TG-2503.0l, Elbi Electric and Lighting, Bragadiru, Romania) with the emission spectrum presented in
Figure S5 in supporting material file. The distance between the light source (lamp) and the reactor was 30 cm. Deionized water was used to prepare the dye solution. In all photocatalytic measurements, the following experimental conditions were employed: initial dye concentration=10 mg/L for all dyes, catalyst dosage = 0.4 g/L (the catalyst loading that proved previously optimal for reference sample T400), maximum irradiation time = 300 min, T = 23 ± 2 °C and pH 7.0 ± 0.2. The pH was recorded for the initial and final solutions and was found to be 7 in both cases. Initially, the dye solution and the catalyst were continuously magnetically stirred (500 rotation/min) in the absence of light for 30 min (time needed to establish an adsorption equilibrium). After this, the solution was irradiated with visible light for 5 hours, and during photodegradation, a quantity of 3 ml of the solution was extracted at the given time intervals to assess the change in the concentration. All absorption spectra for collected samples were measured after 12 h of settling when the catalyst was completely found as sediment. Absorption spectra were recorded using UV–Vis spectrophotometer SPECORD 210
+ (Analytik Jena gmbh, Jena, Germany).
The photocatalytic efficiency (%) for degradation of the dyes was evaluated using the following relationship [
13]:
where C
0 = initial dye concentration (mg/L); C
e = the dye concentration after irradiation time (mg/L).
3.5. Antimicrobial Activity
The antimicrobial activity of the materials was determined by disk diffusion assay against Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922. Both microorganisms were stored at −80 °C in 20–40% glycerol. The bacterial strains were refreshed in tryptic soy agar (TSA) at 36 ± 1 °C for 24 h. Microbial suspensions were prepared with these cultures in sterile solutions to obtain turbidity optically comparable to that of 0.5 McFarland standards.
Volumes of 0.3 ml from each inoculum were spread on the Petri dishes. The sterilized paper disks were placed on plates and an aliquot (20 µL) of the samples with a concentration of 10 mg/ml was added. To evaluate the antimicrobial properties, the growth inhibition was measured under standard conditions after 24 h of incubation at 36 ± 1 °C under solar light illumination. All tests were carried out in triplicate to verify the results. After incubation, the diameters of inhibition zones were measured by using Image J version 1.53e software (Madison, WI, USA), and were expressed as the mean ± standard deviation (SD) performed with XLSTAT software Ecology version 2019.4.1 software (New York, NY, USA) [
49,
50].
3.6. Determination of Minimum Inhibitory Concentration (MIC)
The concentration of a sample that prevented the growth of bacteria is in general recognized as minimum inhibitory concentration (MIC). This was investigated by a broth dilution method, which was performed in 96-well microtiter plates using the resazurin reduction assay concept [
49]. Bacterial culture grown to log phase was adjusted to 1 × 10
8 cells/mL in Muller–Hinton (MH) broth. Inoculants of 50 µL were mixed with 50 µL of serial dilutions of samples and were subsequently incubated at 36 ± 1 °C for 24 h. Resazurin was prepared at 0.015% by dissolving 0.015 g, vortexed and filter sterilized (0.22 µm filter) and stored at 4 °C for a maximum of 2 weeks after preparation. After incubation for 24 h at 36 ± 1 °C, resazurin (0.015%) was added to all wells (20 µL per well), and these were further incubated for 2–4 h more. MIC was determined by reading the fluorescence at λ
ex = 575 nm and λ
em = 590 nm with FLUOstar Omega Microplate Reader (BMG LABTECH, Ortenberg, Germany). To determine accurately the MIC values, the experiments were performed in triplicate.