Apiose-Relevant Glycosidases
Abstract
:1. Introduction
2. General Remarks on Glucosidases
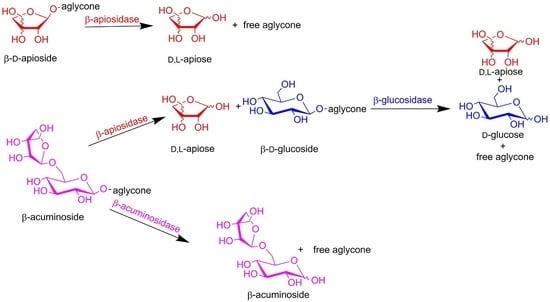
3. Glycosidases in Processing Apiose-containing Saccharides and Glycosides
3.1. β-d-apiofuranosidase (β-apiosidase)
3.1.1. Sources
3.1.2. Purification and Characterization of Apiosidases
3.1.3. Substrate Specificity and Assaying of β-Apiosidases
3.2. Acuminosidases
3.2.1. Sources and Substrate Specificity of β-Acuminosidases
3.2.2. Assay of Acuminosidases
3.2.3. Purification and Characterization of Acuminosidases
3.2.4. Computational and Structural Analysis of Known Diglycosidases and Comparison with Endo-Apiosidase
4. Potential Applications of Apiose-Relevant Glycosidases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pičmanová, M.; Møller, B.L. Apiose: One of nature’s witty games. Glycobiology 2016, 26, 430–442. [Google Scholar] [CrossRef] [Green Version]
- Darvill, A.G.; McNeil, M.; Albersheim, P. Structure of plant cell walls: VIII. A new pectic polysaccharide. Plant Physiol. 1978, 62, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Nepogodiev, S.A.; Fais, M.; Hughes, D.L.; Field, R.A. Synthesis of apiose-containing oligosaccharide fragments of the plant cell wall: Fragments of rhamnogalacturonan-II side chains A and B, and apiogalacturonan. Org. Biomol. Chem. 2011, 9, 6670–6684. [Google Scholar] [CrossRef] [PubMed]
- Duff, R. The occurrence of apiose in Lemna (duckweed) and other angiosperms. Biochem. J. 1965, 94, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Toumy, S.A.; Omara, E.A.; Nada, S.A.; Bermejo, J. Flavone C-glycosides from Montanoa bipinnatifida stems and evaluation of hepatoprotective activity of extract. J. Med. Plants Res. 2011, 5, 1291–1296. [Google Scholar] [CrossRef]
- Gupta, S.R.; Seshadri, T.R. A study of apiin from the parsley seeds and plant. Proc. Indian Acad. Sci.-Sect. A 1952, 35, 242. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Xu, L.; Hao, S.Y.; Li, Y.; Zhang, Z.Q. HPLC method determination of isoliquiritin apioside and isoliquiritin in rat plasma for application in pharmacokinetic study after an oral administration of Zhigancao extract. J. Anal. Methods Chem. 2012, 1, 364013. [Google Scholar] [CrossRef]
- Kernan, M.R.; Amarquaye, A.; Chen, J.L.; Chan, J.; Sesin, D.F.; Parkinson, N.; Ye, Z.; Barrett, M.; Bales, C.; Stoddart, C.A.; et al. Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea. J. Nat. Prod. 1998, 61, 564–570. [Google Scholar] [CrossRef]
- Koike, K.; Li, W.; Liu, L.; Hata, E.; Nikaido, T. New phenolic glycosides from the seeds of Cucurbita moschata. Chem. Pharm. Bull. 2005, 53, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Koike, K.; Tatsuzaki, M.; Koide, A.; Nikaido, T. Cucurbitosides F-M, acylated phenolic glycosides from the seeds of Cucurbita pepo. J. Nat. Prod. 2005, 68, 1754–1757. [Google Scholar] [CrossRef]
- Voirin, S.; Baumes, R.; Bayonove, C.; M’Bairaroua, O.; Tapiero, C. Synthesis and NMR spectral properties of grape monoterpenyl glycosides. Carbohydr. Res. 1990, 207, 39–56. [Google Scholar] [CrossRef]
- Wu, B.; Takahashi, T.; Kashiwagi, T.; Tebayashi, S.; Kim, C.S. New flavonoid glycosides from the leaves of Solidago altissima. Chem. Pharm. Bull. 2007, 55, 815–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuankhayan, P.; Hua, Y.; Svasti, J.; Sakdarat, S.; Sullivan, P.A.; Ketudat Cairns, J.R. Purification of an isoflavonoid 7-O-beta-apiosyl-glucoside beta-glycosidase and its substrates from Dalbergia nigrescens Kurz. Phytochemistry 2005, 66, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sung, S.H.; Kim, Y.C. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J. Nat. Prod. 2005, 68, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Jia, X. Two new coumarin biosides from Angelica dahurica. Chem. Nat. Compd. 2008, 44, 692–695. [Google Scholar] [CrossRef]
- Lee-Juian, L.; Long, Z.L.; Ruangrungsi, N.; Cordell, G.A. 3-Hydroxycoumarin glycosides from Alyxia reinwardti var. Lucida. Phytochemistry 1993, 34, 825–830. [Google Scholar] [CrossRef]
- Imaseki, H.; Yamamoto, T. A furcatin hydrolyzing glycosidase of Viburnum furcatum Blume. Arch. Biochem. Biophys. 1961, 92, 467–474. [Google Scholar] [CrossRef]
- Ahn, Y.O.; Mizutani, M.; Saino, H.; Sakata, K. Furcatin hydrolase from Viburnum furcatum Blume is a novel disaccharide-specific acuminosidase in glycosyl hydrolase family 1. J. Biol Chem. 2004, 279, 23405–23414. [Google Scholar] [CrossRef] [Green Version]
- Günata, Z.; Bitteur, S.; Brillouet, J.-M.; Bayonove, C.; Cordonnier, R. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Günata, Z.; Blondeel, C.; Vallier, M.J.; Lepoutre, J.P.; Sapis, J.C.; Watanabe, N. An endoglycosidase from grape berry skin of cv. M. Alexandria hydrolyzing potentially aromatic disaccharide glycosides. J. Agric. Food Chem. 1998, 46, 2748–2753. [Google Scholar] [CrossRef]
- Ogawa, K.; Ijima, Y.; Guo, W.; Watanabe, N.; Usui, T.; Dong, S.; Tong, Q.; Sakata, K. Purification of a β-primeverosidase concerned with alcoholic aroma formation in tea leaves (cv. Shuixian) to be processed to Oolong tea. J. Agric. Food Chem. 1997, 45, 877–882. [Google Scholar] [CrossRef]
- Günata, Z.; Dugelay, I.; Vallier, M.J.; Sapis, J.C.; Bayonove, C. Multiple forms of glycosidases in an enzyme preparation from Aspergillus niger: Partial characterization of a β-apiosidase. Enzym. Microb. Technol. 1997, 21, 39–44. [Google Scholar] [CrossRef]
- Guo, W.; Salmon, J.M.; Baumes, R.; Tapiero, C.; Günata, Z. Purification and some properties of an Aspergillus niger β-apiosidase from an enzyme preparation hydrolyzing aroma precursors. J. Agric. Food Chem. 1999, 47, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Sarry, J.-E.; Günata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Bojarová-Fialová, P.; Křen, V. Enzymatic approaches to O-glycoside introduction: Glycosidases. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2007; pp. 453–487. [Google Scholar] [CrossRef]
- Dupin, I.; Gunata, Z.; Sapis, J.C.; Bayonove, C.; Mbairaroua, O.; Tapiero, C. Production of beta-apiosidase by Aspergillus-niger–partial purification, properties, and effect on terpenyl apiosylglucosides from grape. J. Agric. Food Chem. 1992, 40, 1886–1891. [Google Scholar] [CrossRef]
- Kis, P.; Potocká, E.; Mastihuba, V.; Mastihubová, M. Efficient chemoenzymatic synthesis of 4-nitrophenyl β-d-apiofuranoside and its use in screening of β-d-apiofuranosidases. Carbohydr. Res. 2016, 430, 48–53. [Google Scholar] [CrossRef]
- Karkeszová, K.; Illeová, V.; Kis, P.; Mastihuba, V.; Polakovič, M. Apiin-induction of β-apiosidase production by Aspergillus sp. strains. Acta Chim. Slov. 2020, 13, 72–76. [Google Scholar] [CrossRef]
- Sarry, J.E.; Grimplet, J.; Sommerer, N.; Vallier, M.J.; Pradal, M.; Mondolot, L.; Andary, C.; Günata, Z.; Romieu, C. Combined mass mapping and biochemical characterization of grape β-glycosidase-enriched extract. Protein J. 2008, 27, 258–266. [Google Scholar] [CrossRef]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Luis, A.S.; Baslé, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70. [Google Scholar] [CrossRef]
- Mastihuba, V.; Karnisova Potocka, E.; Uhliarikova, I.; Kis, P.; Kozmon, S.; Mastihubova, M. Reaction mechanism of beta-apiosidase from Aspergillus aculeatus. Food Chem. 2019, 274, 543–546. [Google Scholar] [CrossRef]
- Mizutani, M.; Nakanishi, H.; Ema, J.; Ma, S.J.; Noguchi, E.; Inohara-Ochiai, M.; Fukuchi-Mizutani, M.; Nakao, M.; Sakata, K. Cloning of beta-primeverosidase from tea leaves, a key enzyme in tea aroma formation. Plant Physiol. 2002, 130, 2164–2176. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferro, L.S.; Breccia, J.D. Functional and biotechnological insights into diglycosidases. Biocatal. Biotransformation 2011, 29, 103–112. [Google Scholar] [CrossRef]
- Hosel, W.; Barz, W. Beta-glucosidases from Cicer arietinum L. Purification and properties of isoflavone-7-O-glucoside-specific beta-glucosidases. Eur. J. Biochem. 1975, 57, 607–616. [Google Scholar] [CrossRef]
- Chuankhayan, P.; Rimlumduan, T.; Tantanuch, W.; Mothong, N.; Kongsaeree, P.T.; Metheenukul, P.; Svasti, J.; Jensen, O.N.; Ketudat Cairns, J.R. Functional and structural differences between isoflavonoid β-glycosidases from Dalbergia sp. Arch. Biochem. Biophys. 2007, 468, 205–216. [Google Scholar] [CrossRef]
- Baumgertel, A.; Grimm, R.; Eisenbeiss, W.; Kreis, W. Purification and characterization of a flavonol 3-O-beta-heterodisaccharidase from the dried herb of Fagopyrum esculentum Moench. Phytochemistry 2003, 64, 411–418. [Google Scholar] [CrossRef]
- Mazzaferro, L.S.; Breccia, J.D. Quantification of hesperidin in citrus-based foods using a fungal diglycosidase. Food Chem. 2012, 134, 2338–2344. [Google Scholar] [CrossRef]
- Daiyasu, H.; Saino, H.; Tomoto, H.; Mizutani, M.; Sakata, K.; Toh, H. Computational and experimental analyses of furcatin hydrolase for substrate specificity studies of disaccharide-specific glycosidases. J. Biochem. 2008, 144, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A. 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Gunata, Z.; Bitteur, S.; Baumes, R.; Brillouet, J.-M.; Tapiero, C.; Bayonove, C.; Cordonnier, R. Process for Obtaining Aroma Components and Aromas from Their Precursors of a Glycosidic Nature, and Aroma Components and Aromas Thereby Obtained. Patent No. EP0416713B2, 21 September 1990. [Google Scholar]
- Karnišová Potocká, E.; Mastihubová, M.; Mastihuba, V. Transrutinosylation of tyrosol by flower buds of Sophora japonica. Food Chem. 2021, 336, 127674. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Ohno, F.; Yamauchi, Y.; Kato, M.; Makabe, H.; Nakamura, S. Enzymatic synthesis of novel phenol acid rutinosides using rutinase and their antiviral activity in vitro. J. Agric. Food Chem. 2013, 61, 9617–9622. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, L.; Piñuel, L.; Minig, M.; Breccia, J.D. Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid β-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria. Arch. Microbiol. 2010, 192, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, L.S.; Piñuel, L.; Erra-Balsells, R.; Giudicessi, S.L.; Breccia, J.D. Transglycosylation specificity of Acremonium sp. α-rhamnosyl-β-glucosidase and its application to the synthesis of the new fluorogenic substrate 4-methylumbelliferyl-rutinoside. Carbohydr. Res. 2012, 347, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tsuruhami, K.; Mori, S.; Amarume, S.; Saruwatari, S.; Murata, T.; Hirakake, J.; Sakata, K.; Usui, T. Isolation and characterization of a β-primeverosidase-like enzyme from Penicillium multicolor. Biosci. Biotech. Biochem. 2006, 70, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Koseki, T.; Ishikawa, M.; Kawasaki, M.; Shiono, Y. β-Diglycosidases from microorganisms as industrial biocatalysts: Biochemical characteristics and potential applications. Appl. Microbiol. Biotechnol. 2018, 102, 8717–8723. [Google Scholar] [CrossRef] [PubMed]








| Enzyme Preparation | Producer | Microbial Source | Original Designation | References |
|---|---|---|---|---|
| Rapidase AR 2000 (Klerzyme 200) | DSM | Aspergillus niger | Pectinases, polygalacturonases, β-glucanases | [22,24,26,27] |
| Pectinase 263 | Gist Brocades | A. niger | Pectinases, polygalacturonases | [22,24,26] |
| Pectolase 3PA 400 | Grinsted | A. niger | Pectinases, polygalacturonases | [22,24,26] |
| Ultrazym 100 | Ciba-Geigy | A. niger | Pectinases, polygalacturonases β-glucanases | [22,24,26,27] |
| Lallzyme BETA | Lallemand | A. niger | Pectinases, polygalacturonases, β-glucanases | [27] |
| Rapidase Expression | DSM | A. niger | Pectinases, polygalacturonases, β-glucanases | [27] |
| α-galactosidase DS | Amano | A. niger | α-Galactosidase | [27] |
| Lallzyme Cuvée Blanc | Lallemand | A. niger | β-Glucosidase | [27] |
| Lipolyve AN | Lyven | A. niger | Lipases | [27] |
| Enzeco Lipase | Enzyme Development Corporation | A. niger | Lipases | [27] |
| Lipase A | Amano | A. niger | Lipases | [27] |
| Viscozyme L | Novozymes | A. aculeatus | β-Glucanases, cellulases | [27] |
| Novozym 188 | Novozymes | A. niger | β-Glucanases, cellulases | [27] |
| Pectinex UF | Novozymes | A. aculeatus | Pectinases, polygalacturonases | [27] |
| Ultrazym AFP | Novozymes | Aspergillus sp. | Pectinases, polygalacturonases | [27] |
| TP714L | Biocatalysts | Aspergillus sp. | Pectinases, polygalacturonases | [27] |
| Peclyve LVG | Lyven | A. niger | Pectinases, polygalacturonases | [27] |
| Depol 692L | Biocatalysts | Aspergillus sp. & Trichoderma | β-Glucanases, cellulases | [27] |
| Source | Molecular Mass [kDa] | Km 1 [mM] | Vmax 1 [nkat/mg] | Optimal pH | Optimal Temperature [°C] | pI |
|---|---|---|---|---|---|---|
| A. niger [26] | 38 | 16 | 0.192 | 5.6 | 50 | - |
| Klerzyme 200 [22] | 84 | 3.3 | 216 | 5.0–6.0 | 50–60 | - |
| Klerzyme 200 [23] | 120 | 4.16 | 2460 | 5.0 | 40 | 3.5 |
| B. thetaiotaomicron [30] | - | - | - | 6.5–7.5 | 37 | - |
| Source | Tested Activity | |
|---|---|---|
| Confirmed | Zero or Negligible | |
| Viburnum furcatum [17] | Furcatin n-Propyl β-acuminoside n-Butyl β-acuminoside Ethyleneglycol monomethylether β-acuminoside | Apiin Rutin |
| Viburnum furcatum [18] | Furcatin 4-Allylphenyl β-d-glucopyranoside pNP β-primeveroside 2-Phenylethyl β-primeveroside | Vicianin pNP β-gentiobioside pNP β-d-glucopyranoside Amygdalin Prunasin 2-phenylethyl-β-gentiobioside 2-Phenylethyl β-d-glucoside |
| Cicer arietinum L. [34] | Biochanin A 7-O-β-d-glucoside Biochanin A 7-O-β-apioglucoside Formononetin 7-O-β-d-glucoside 2-Methyl-4’-nitro-isoflavone 7-O-β-d-glucoside Apiigenin 7-O-β-d-glucoside 4Mum β-d-glucopyranoside pNP β-d-glucopyranoside pNP β-d-galactopyranoside oNP β-d-glucopyranoside Salicin | Formononetin 7-O-β-cellobioside Genistein 4’-O-β-d-glucoside Apigenin 7-O-β-apioglucoside Kaempferol 3-O-β-d-glucoside Kaempferol 3-O-β-apioglucoside Quercetin 3-O-β-rutinoside 4Mum β-d-glucuronide 4Mum β-d-N-acetylglucosamide pNP α-d-glucoside Phloridzin Cellobiose |
| Dalbergia nigescens Kurz [13] | Dalpatein β-acuminoside Dalnigrein β-acuminoside Genistin (genistein 7-O-Glcp) Daidzin (daidzein 7-O-Glcp) pNP β-d-fucoside pNP β-d-glucopyranoside | - |
| Vitis vinifera [20] | 2-phenylethyl β-rutinoside pNP β-rutinoside Neryl β-rutinoside Linalyl β-rutinoside Neryl 3-O-α-l-arabinofuranosyl- (1→6)-β-d-glucopyranoside Linaloyl 3-O-α-l-arabinofuranosyl- (1→6)-β-d-glucopyranoside Geranyl β-acuminoside Linalyl β-acuminoside 4Mum β-vicianoside Eugenyl β-primeveroside | Peltatoside 1 |
| Source | Molecular Mass [kDa] | Oligomer | Km [mM] | Vmax [nkat/mg] | Optimal pH | Optimal Temperature [°C] |
|---|---|---|---|---|---|---|
| Cicer arietinum L. [34] | 68 (subunit) | Dimer | 2 1 0.2 2 | - | 7.0–7.5 (add. minor 4.5–5.0) | 45 |
| Viburnum furcatum Blume [17] | - | - | - | - | 5.8-6.3 | Below 40 |
| Viburnum furcatum Blume [18] | 56 61 (recombinant) | Monomer | 2.2 3 | - | 5 | 40 |
| Dalbergia nigrescens Kurz [13] | 62–63 (subunit) | Tetramer | 14.7 1 0.5 4 (0.7) 4 | 66.6 1 | 5.0–6.0 | 65 |
| Vitis vinifera endoglycosidase [20] | 58 | - | 1.69 5 | 275 5 | 4–5 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karnišová Potocká, E.; Mastihubová, M.; Mastihuba, V. Apiose-Relevant Glycosidases. Catalysts 2021, 11, 1251. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101251
Karnišová Potocká E, Mastihubová M, Mastihuba V. Apiose-Relevant Glycosidases. Catalysts. 2021; 11(10):1251. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101251
Chicago/Turabian StyleKarnišová Potocká, Elena, Mária Mastihubová, and Vladimír Mastihuba. 2021. "Apiose-Relevant Glycosidases" Catalysts 11, no. 10: 1251. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101251