Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis
Abstract
:1. Introduction
2. Results
2.1. Synthesis of the Investigated Naphthalimides
2.1.1. Synthesis of 6-Bromo-2-(naphthalen-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione
2.1.2. Synthesis of 2-(Naphthalen-1-yl)-6-(pyrrolidin-1-yl)-1H-benzo[de]isoquinoline1,3(2H)-dione (Napht-1)
2.1.3. Synthesis of 2-(Naphthalen-1-yl)-6-(piperidin-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Napht-2)
2.1.4. Synthesis of 6-Morpholino-2-(naphthalen-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Napht-3)
2.1.5. Synthesis of 6-(4-Methylpiperazin-1-yl)-2-(naphthalen-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Napht-4)
2.1.6. Synthesis of 6-(Xexylamino)-2-(naphthalen-1-yl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Napht-5)
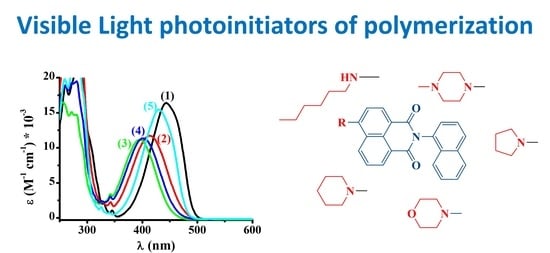
2.2. Study of the UV–Visible Spectra of the Different Naphthalimides
2.3. Photopolymerization Experiments Using Different Monomers
2.3.1. Free Radical Photopolymerization of Acrylate Monomer (TA)
2.3.2. Cationic Polymerization
2.3.3. Interpenetrating Polymer Network (IPN) Synthesis
2.4. Photocomposite Synthesis Using Napht/Iod/NPG (0.05%/1%/1% w/w/w) System
2.5. Direct Laser Write (DLW)
3. Discussion: Photophysical/Chemical Properties of Naphthalimide Dyes
3.1. Steady State Photolysis of Dyes Based Naphthalimides
3.2. Reactivity of the Excited State for the Naphthalimide Compounds
3.2.1. Singlet State Properties: Time Resolved Fluorescence
3.2.2. Fluorescence Quenching
3.2.3. Chemical Mechanisms
| Napht (hν) → 1,3 Napht | r1 |
| 1,3 Napht + ArI+ → Ar● + ArI + Napht ●+ | r2 |
| NPG + Iod → [NPG-Iod]CTC | r3 |
| [NPG-Iod]CTC → Ar● | r4 |
| 1,3 Napht + NPG → Napht-H● + NPG(-H)● | r5 |
| NPG(-H)● → NPG(-H;-CO2)● + CO2 | r6 |
| NPG(-H;-CO2) ● + Ar2I+ → NPG(-H;-CO2)+ + Ar● + ArI | r7 |
| Napht ●+ + NPG → Napht + NPG+ | r8 |
| Napht-H● + ArI+ → Ar● + ArI + Napht + H+ | r9 |
4. Materials and Methods
4.1. Other Chemicals
4.2. Irradiation Sources
4.3. Free Radical Polymerization and Cationic Polymerization Profile Determination Using Real-Time Fourier Transform Infrared Spectroscopy (RT-FTIR)
4.4. Redox Potentials and Free Energy Change Calculation
4.5. Steady State Photolysis, UV–Visible Absorption, Fluorescence Spectroscopy, and Time-Resolved Fluorescence Spectroscopy Experiments
4.6. Computational Procedure
4.7. Near-UV Conveyor Experiments: Photocomposites Synthesis
4.8. 3D Printing Experiments and Direct Laser Write (DLW)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Baghdachi, J. Functional Polymer Coatings: Principles, Methods, and Applications; Wiley Series on Polymer Engineering and Technology; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Lawrence, J.; O’Neill, F.; Sheridan, J. Photopolymer holographic recording material. Optik 2001, 112, 449–463. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Maier, M.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Klee, J.E.; Lalevée, J. A novel photoinitiating systemproducing germyl radicals for the polymerization of representative methacrylate resins: Camphorqui-none/R3GeH/iodonium salt. Dent. Mater. 2016, 32, 1226–1234. [Google Scholar] [CrossRef]
- Morgan, S.E.; Havelka, K.O.; Lochhead, R.Y. Cosmetic Nanotechnology: Polymers and Colloids in Cosmetics in Person Care; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; p. 961. [Google Scholar]
- Drobny, J.G. Radiation Technology for Polymers; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Xie, C.; Leng, K.; Sheng, J.; Wang, X.; Li, Q.; Song, L.; Liu, L.; Sun, H.; Yu, Q. Preparation of poly(methyl methacrylate) micro-spheres via photopolymerization initiated by LED light source. Colloid. Polym. Sci. 2020, 298, 1285–1291. [Google Scholar] [CrossRef]
- Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Seeing the Light: Advancing Materials Chemistry through Photopol-ymerization. Angew. Chem. Int. Ed. 2019, 58, 5170–5189. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yagci, Y. Photoinduced metal-free atom transfer radical polymerizations: State-of-the-art, mechanistic aspects and applications. Polym. Chem. 2018, 9, 1757–1762. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2019, 11, 1111–1121. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Pertici, V.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Naphthalimide-Based Dyes as Photoinitiators under Visible Light Irradiation and their Applications: Photocomposite Synthesis, 3D printing and Polymerization in Water. ChemPhotoChem 2021, 5, 476–490. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Zahouily, S.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Ibrahim-Ouali, M.; Gigmes, D.; Hoffmann, N.; Dumur, F.; Lalevée, J. New 1,8-Naphthalimide Derivatives as Photoinitiators for Free-Radical Polymerization Upon Visible Light. Catalysts 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide-tertiary amine deriva-tives as blue-light-sensitive photoinitiators. ChemPhotoChem 2018, 2, 481–489. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide Derivatives: Substituent Effects on the Photoinitiating Ability in Polymerizations under Near UV, Purple, White and Blue LEDs (385, 395, 405, 455, or 470 nm). Macromol. Chem. Phys. 2015, 216, 1782–1790. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structure design of naph-thalimide derivatives: Towards versatile photo-initiators for near UV/Visible LEDs, 3D printing and water-soluble photoiniti-ating systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on naphthalic anhydrides and 1,8-naphthalimide-based photoinitiators of polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. N-[2-(dimethylamino)ethyl]-1,8-naphthalimide derivatives as photo-initiators under LEDs. Polym. Chem. 2018, 9, 994–1003. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New role of aminothiazonaphthalimide deriv-atives: Outstanding photoinitiators for cationic and radical photopolymerizations under visible LEDs. RSC Adv. 2016, 6, 48684–48693. [Google Scholar] [CrossRef]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel naphthalimide-amine based photoinitiators operating under violet and blue LEDs and us-able for various polymerization reactions and synthesis of hydrogels. Polym. Chem. 2016, 7, 418–429. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevéee, J. Aminothiazonaphthalic anhydride derivatives as photoinitiators for violet/blue LED-Induced cationic and radical photopolymerizations and 3D-Printing resins. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 1189–1196. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalic anhydride derivatives: Structural effects on their initiating abilities in radical and/or cationic photopolymerizations under visible light. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2860–2866. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide-phthalimide derivative based photoinitiating systems for polymerization reactions under blue lights. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 665–674. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A benzophenone-naphthalimide derivative as versatile photoinitiator of polymerization under near UV and visible lights. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 445–451. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue light sensitive dyes for various photopolymeriza-tion reactions: Naphthalimide and naphthalic anhydride derivatives. Macromolecules 2014, 47, 601–608. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Tehfe, M.-A.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide based methacrylated photoinitiators in radical and cationic photopolymerization under visible light. Polym. Chem. 2013, 4, 5440–5448. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Recent Advances in Photoinduced Polymerization Reactions under 400−700 nm Light. Photochemistry 2014, 42, 215–232. [Google Scholar]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the Quest of New Photocatalysts for Polymerization Reactions. Accounts Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymeri-zation Reactions. ChemCatChem. 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.P.; Lalevéee, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 702–713. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Cationic and Thiol–Ene Photopolymerization upon Red Lights Using Anthraquinone Derivatives as Photoinitiators. Macromolecules 2013, 46, 6744–6750. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Vidal, L.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structural Effects in the Indanedione Skeleton for the Design of Low Intensity 300–500 nm Light Sensitive Initiators. Macromolecules 2013, 47, 26–34. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient Dual Radi-cal/CationicPhotoinitiator under Visible Light: A New Concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A Novel Photopolymerization Initiating System Based on an Iridium Complex Photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, X.; Su, D.; Zhang, Y.; Li, P.; Lu, S.; Gong, Y.; Zhang, W.; Tang, B. Simultaneous Fluorescence Imaging Reveals N-Methyl-d-aspartic Acid Receptor Dependent Zn2+/H+ Flux in the Brains of Mice with Depression. Anal. Chem. 2020, 92, 4101–4107. [Google Scholar] [CrossRef] [PubMed]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods. In Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian Inc.: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, J.R.E.; Burant, J.C.; et al. Gaussian 03, Revision B-2; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Goubard, F.; Bui, T.-T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; et al. Azahelicenes as visible light photoinitiators for cationic and radical polymerization: Preparation of pho-to-luminescent polymers and use in high performance LED projector 3D printing resins. J. Polym. Sci. A Polym. Chem. 2017, 55, 1189–1199. [Google Scholar] [CrossRef]









| λmax (nm) | εmax (M−1·cm−1) | ε405 nm (M−1·cm−1) | ε455 nm(M−1·cm−1) | ε470 nm (M−1·cm−1) | |
|---|---|---|---|---|---|
| Napht-1 | 444 | 16,400 | 6770 | 15,340 | 11,400 |
| Napht-2 | 414 | 11,200 | 11,250 | 4450 | 1750 |
| Napht-3 | 396 | 11,150 | 10,500 | 890 | 60 |
| Napht-4 | 403 | 11,300 | 11,300 | 1950 | 500 |
| Napht-5 | 430 | 14,200 | 10,360 | 11,000 | 4330 |
| Two-Component PIS: Napht/Iod in Thick Samples | Two-Component PIS: Napht/Iod in Thin Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| @405 nm | @455 nm | @405 nm | @455 nm | |||||||||
| 0.05% | 0.1% | 0.2% | 0.05% | 0.1% | 0.2% | 0.05% | 0.1% | 0.2% | 0.05% | 0.1% | 0.2% | |
| Napht-1 | 79% | 70% | 54% | 81% | 72% | 62% | 55% | 53% | 72% | 50% | 65% | 66% |
| Napht-2 | 80% | 79% | 62% | 80% | 76% | 63% | 55% | 67% | 70% | 44% | 60% | 72% |
| Napht-3 | 80% | 37% | 28% | 80% | 90% | 36% | 61% | 69% | 72% | 60% | 56% | 69% |
| Napht-4 | 83% | 70% | 54% | 74% | 79% | 72% | 46% | 56% | 68% | 29% | 43% | 63% |
| Napht-5 | 79% | 67% | 62% | 85% | 65% | 60% | 50% | 69% | 70% | 50% | 71% | 69% |
| Three-Component PISs: Napht/Iod/NPG (0.05%/1%/1% w/w/w) | ||||
|---|---|---|---|---|
| Thick Sample | Thin Sample | |||
| @405 nm | @455 nm | @405 nm | @455 nm | |
| Napht-1 | 88% | 81% | 77% | 77% |
| Napht-2 | 90% | 88% | 77% | 78% |
| Napht-3 | 88% | 91% | 80% | 78% |
| Napht-4 | 89% | 90% | 79% | 75% |
| Napht-5 | 89% | 76% | 72% | 78% |
| Three-Component PISs: Napht-N/Iod/NVK | ||
|---|---|---|
| Thick Sample | Thin Sample | |
| Napht-1 | 23% | 50% |
| Napht-2 | 30% | 40% |
| Napht-3 | 42% | 45% |
| Napht-4 | 12% | 56% |
| Napht-5 | 27% | 37% |
| Two-Component PIS: Napht/Iod, TA/EPOX (50%/50%) | ||||
|---|---|---|---|---|
| Thick Sample (1.4 mm) | Thin Sample (25 μm) | |||
| @405 nm | @455 nm | @405 nm | @455 nm | |
| Napht-1 | 90%/10% | 86%/40% | 88%/30% | 80%/35% |
| Napht-2 | 86%/20% | 92%/35% | 88%/29% | 82%/32% |
| Napht-3 | 90%/20% | 77%/25% | 87%/48% | 81%/35% |
| Napht-4 | 85%/40% | 83%/15% | 82%/54% | 66%/50% |
| Napht-5 | 88%/18% | 80%/10% | 84%/32% | 78%/36% |
| Eox (eV) | ES1 (eV) | ΔGS1 (eV) | Ksv | Φ (PI/Iod) | Lifetime S1 (ns) | |
|---|---|---|---|---|---|---|
| Napht-1 | 1.05 | 2.58 | −0.83 | 64.7 | 0.55 | 8.53 |
| Napht-2 | 1.08 | 2.62 | −0.84 | 48.5 | 0.55 | 8.62 |
| Napht-3 | 1.23 | 2.70 | −0.77 | 52 | 0.52 | 8.28 |
| Napht-4 | 1.14 | 2.67 | −0.83 | 0.58 | ||
| Napht-5 | 1.13 | 2.61 | −0.78 | 63 | 0.56 | 9.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis. Catalysts 2021, 11, 1269. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111269
Rahal M, Graff B, Toufaily J, Hamieh T, Ibrahim-Ouali M, Dumur F, Lalevée J. Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis. Catalysts. 2021; 11(11):1269. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111269
Chicago/Turabian StyleRahal, Mahmoud, Bernadette Graff, Joumana Toufaily, Tayssir Hamieh, Malika Ibrahim-Ouali, Frédéric Dumur, and Jacques Lalevée. 2021. "Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis" Catalysts 11, no. 11: 1269. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111269