Enhancement of Biological Pretreatment on Rice Straw by an Ionic Liquid or Surfactant
Abstract
:1. Introduction
2. Results and Discussion
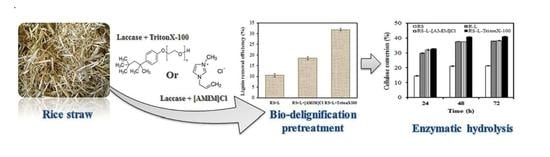
2.1. Effect of Laccase and Laccase with IL or Surfactant Pretreatment on Rice Straw Delignification
2.2. Effect of Laccase and Laccase with IL or Surfactant Pretreatment on the Rice Straw Composition
2.3. Effect of Laccase and Laccase with IL or Surfactant Pretreatment on Enzymatic Saccharification Based on Different Strategies
2.4. SEM Images
2.5. Characterization of the Untreated and Pretreated Rice Straw
2.6. AFM
3. Materials and Methods
3.1. Materials
3.2. Rice Straw Pretreatment
3.3. Enzymatic Saccharification
3.4. SEM Analysis
3.5. FTIR Analysis
3.6. XRD Analysis
3.7. AFM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerbens-Leenes, P.W. Green, Blue and Grey Bioenergy Water Footprints, a Comparison of Feedstocks for Bioenergy Supply in 2040. Environ. Process. 2018, 5, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Vasco-Correa, J.; Li, Y. Solid-state anaerobic digestion of fungal pretreated Miscanthus sinensis harvested in two different seasons. Bioresour. Technol. 2015, 185, 211–217. [Google Scholar] [CrossRef]
- Zaky, A.S.; Carter, C.E.; Meng, F.; French, C.E. A Preliminary Life Cycle Analysis of Bioethanol Production Using Seawater in a Coastal Biorefinery Setting. Processes 2021, 9, 1399. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bio-ethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Srivastava, M.; Shukla, A. Environmental sustainability of bioethanol production from rice straw in India: A review. Renew. Sustain. Energy Rev. 2016, 54, 202–216. [Google Scholar] [CrossRef]
- Lavarda, G.; Morales-delaRosa, S.; Centomo, P.; Campos-Martin, J.M.; Zecca, M.; Fierro, J.L.G. Gel-Type and Macroporous Cross-Linked Copolymers Functionalized with Acid Groups for the Hydrolysis of Wheat Straw Pretreated with an Ionic Liq-uid. Catalysts 2019, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Charisteidis, I.; Lazaridis, P.; Fotopoulos, A.; Pachatouridou, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Triantafyllidis, K. Cat-alytic Fast Pyrolysis of Lignin Isolated by Hybrid Organosolv—Steam Explosion Pretreatment of Hardwood and Softwood Biomass for the Production of Phenolics and Aromatics. Catalysts 2019, 9, 935. [Google Scholar] [CrossRef] [Green Version]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly celluloly-tic fungus Trichoderma viride. Waste Manage 2015, 43, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Anderson, J.A.; Aulenta, F.; McCue, A.; Paton, G. The potential of microbial processes for lignocellulosic biomass conversion to ethanol: A review. J. Chem. Technol. Biotechnol. 2015, 90, 366–383. [Google Scholar] [CrossRef]
- Aracri, E.; Vidal, T. Enhancing the effectiveness of a laccase–TEMPO treatment has a biorefining effect on sisal cellulose fibres. Cellulose 2012, 19, 867–877. [Google Scholar] [CrossRef]
- Ibrahim, V.; Mendoza, L.; Mamo, G.; Hatti-Kaul, R. Blue laccase from Galerina sp.: Properties and potential for Kraft lignin demethylation. Process. Biochem. 2011, 46, 379–384. [Google Scholar] [CrossRef]
- Mustafa, A.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, H. Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour. Technol. 2012, 118, 8–12. [Google Scholar] [CrossRef]
- Yu, H.; Du, W.; Zhang, J.; Ma, F.; Zhang, X.; Zhong, W. Fungal treatment of cornstalks enhances the delignification and xylan loss during mild alkaline pretreatment and enzymatic digestibility of glucan. Bioresour. Technol. 2010, 101, 6728–6734. [Google Scholar] [CrossRef]
- Elgharbawy, A.; Alam, Z.; Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem. Eng. J. 2016, 109, 252–267. [Google Scholar] [CrossRef]
- Chang, K.-L.; Chen, X.-M.; Han, Y.-J.; Wang, X.-Q.; Potprommanee, L.; Ning, X.-A.; Liu, J.-Y.; Sun, J.; Peng, Y.-P.; Sun, S.-Y.; et al. Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour. Technol. 2016, 214, 371–375. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wang, Y.; Sun, J.; Huang, P.; Chang, K. Effect of ultrasound on ionic liquid-hydrochloric acid pretreatment with rice straw. Biomass Convers. Biorefinery 2021, 11, 1749–1757. [Google Scholar] [CrossRef]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef]
- Chang, K.-L.; Han, Y.-J.; Wang, X.-Q.; Chen, X.-M.; Leu, S.-Y.; Liu, J.-Y.; Peng, Y.-P.; Liao, Y.-L.; Potprommanee, L. The effect of surfactant-assisted ultrasound-ionic liquid pretreatment on the structure and fermentable sugar production of a water hyacinth. Bioresour. Technol. 2017, 237, 27–30. [Google Scholar] [CrossRef]
- Virak, S.; Chang, K.L.; Phitsuwanb, P.; Ratanakhanokchaib, K.; Dong, C.D. Effect of microwave-assisted ionic liquid/acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour. Technol. 2019, 293, 121929. [Google Scholar]
- Naushad, M.; Alothman, Z.; Khan, A.B.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef]
- Moirangthem, K.; Zaky, A.S.; Tucker, G.A. Microwave subcritical water pre-treatment and enzymatic hydrolysis of geo-graphical identification (GI) tag Indian black rice (Chakhao Poireiton) straw for fermentable sugar production. Biofuels 2021. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Liu, D.-H. Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: A continued work. J. Chem. Technol. Biotechnol. 2008, 83, 950–956. [Google Scholar] [CrossRef]
- Chang, K.L.; Chen, X.M.; Wang, X.Q.; Han, Y.J.; Potprommanee, L.; Liu, Y.J.; Liao, Y.L.; Ning, X.A.; Sun, Y.; Huang, Q. Im-pact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour. Technol. 2016, 227, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-L.; Wang, X.-Q.; Han, Y.-J.; Deng, H.; Liu, J.-Y.; Lin, Y.-C. Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology. Materials 2018, 11, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtubise, F.G.; Krässig, H. Classification of fine structural characteristics in cellulose by infrared spectroscopy. Anal. Chem. 1960, 32, 177–181. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Zhang, Z.; O’Hara, I.; Doherty, W. Pretreatment of sugarcane bagasse by acid-catalysed process in aqueous ionic liquid solutions. Bioresour. Technol. 2012, 120, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Jones, C.L.; Baker, G.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-J.; Kim, S.-H.; Chung, I.-M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Martinez-Pavetti, M.; Medina, L.; Espinola, M.; Monteiro, M. Study on two eco-friendly surface treatments on Luffa cylindrical for development of reinforcement and processing materials. J. Mater. Res. Technol. 2021, 14, 2420–2427. [Google Scholar] [CrossRef]





| Pretreatment | Lignin (%) | Cellulose (%) | Hemicellulose (%) | Recovered Solids (%) |
|---|---|---|---|---|
| Untreated | 15.79 ± 0.01 | 31.73 ± 0.02 | 23.21 ± 0.03 | 100.00 |
| L | 13.90 ± 0.01 | 38.50 ± 0.02 | 21.67 ± 0.03 | 94.25 |
| L + [AMIM]Cl | 12.87 ± 0.01 | 39.57 ± 0.02 | 21.34 ± 0.04 | 93.91 |
| L + TritonX-100 | 10.77 ± 0.01 | 40.83 ± 0.02 | 19.66 ± 0.04 | 93.23 |
| Pretreatments | LOI (A1424/A896) | TCI (A1368/A2900) | CrI (%) |
|---|---|---|---|
| Untreated | 1.5955 | 1.2423 | 67.85 |
| L | 1.5774 | 1.2282 | 66.17 |
| L+[AMIM]Cl | 1.5746 | 1.1976 | 64.71 |
| L+TritonX-100 | 1.5689 | 1.1947 | 64.58 |
| Pretreatment | Square Roughness (Rq) | Average Roughness (Ra) | Surface Area (Sa, nm2) |
|---|---|---|---|
| Untreated | 4.89 | 3.99 | 1,001,664 |
| L | 12.30 | 9.59 | 1,069,663 |
| L + [AMIM]Cl a | 19.8 | 15.5 | 1,064,632 |
| L + TritonX-100 b | 21.4 | 17.7 | 1,081,602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-L.; Liu, C.-H.; Phitsuwan, P.; Ratanakhanokchai, K.; Lin, Y.-C.; Dong, C.-D.; Lin, M.-H.; Yang, G.C.C. Enhancement of Biological Pretreatment on Rice Straw by an Ionic Liquid or Surfactant. Catalysts 2021, 11, 1274. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111274
Chang K-L, Liu C-H, Phitsuwan P, Ratanakhanokchai K, Lin Y-C, Dong C-D, Lin M-H, Yang GCC. Enhancement of Biological Pretreatment on Rice Straw by an Ionic Liquid or Surfactant. Catalysts. 2021; 11(11):1274. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111274
Chicago/Turabian StyleChang, Ken-Lin, Chun-Hung Liu, Paripok Phitsuwan, Khanok Ratanakhanokchai, Yung-Chang Lin, Cheng-Di Dong, Ming-Hsun Lin, and Gordon C. C. Yang. 2021. "Enhancement of Biological Pretreatment on Rice Straw by an Ionic Liquid or Surfactant" Catalysts 11, no. 11: 1274. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111274