Dual-Modified Cu2S with MoS2 and Reduced Graphene Oxides as Efficient Photocatalysts for H2 Evolution Reaction
Abstract
:1. Introduction
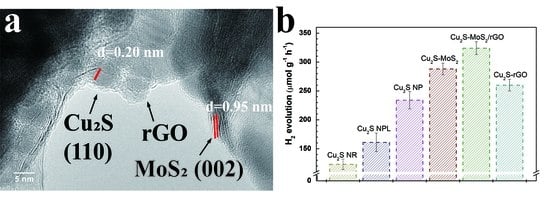
2. Results and Discussion
3. Experimental
3.1. Synthesis of Graphene Oxide (GO)
3.2. Synthesis of 1T-MoS2/rGO Hybrid
3.3. Synthesis of Cu2S Nanostructures and Cu2S-MoS2/rGO Composites
3.4. Characterization and Photocatalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Liu, Y.; Gu, B.; Wei, X.; Xu, G.; Wang, X.; Swihart, M.T.; Yong, K.-T. Recent advances in copper sulphide-based nanoheterostructures. Chem. Soc. Rev. 2019, 48, 4950–4965. [Google Scholar] [CrossRef]
- Nikam, A.N.; Pandey, A.; Fernandes, G.; Kulkarni, S.; Mutalik, S.P.; Padya, B.S.; George, S.D.; Mutalik, S. Copper sulphide based heterogeneous nanoplatforms for multimodal therapy and imaging of cancer: Recent advances and toxicological perspectives. Coord. Chem. Rev. 2020, 419, 213356. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, R.; Guo, C.; Bai, X.; Xu, S.; Wang, L. Fluorine Grafted Cu7S4–Au Heterodimers for Multimodal Imaging Guided Photothermal Therapy with High Penetration Depth. J. Am. Chem. Soc. 2018, 140, 5890–5894. [Google Scholar] [CrossRef]
- Peng, Q.K.; Zhang, S.P.; Yang, H.; Sheng, B.B.; Xu, R.; Wang, Q.S.; Yu, Y. Boosting Potassium Storage Performance of the Cu2S Anode via Morphology Engineering and Electrolyte Chemistry. ACS Nano 2020, 14, 6024–6033. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, W.; Ding, N.; Ji, Y.; Pan, G.; Zhu, J.; Zhou, D.; Wu, Y.; Chen, C.; Song, H. Dual Interfacial Modification Engineering with 2D MXene Quantum Dots and Copper Sulphide Nanocrystals Enabled High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 2003295. [Google Scholar] [CrossRef]
- Srinivas, B.; Kumar, B.G.; Muralidharan, K. Stabilizer free copper sulphide nanostructures for rapid photocatalytic decomposition of rhodamine B. J. Mol. Catal. A Chem. 2015, 410, 8–18. [Google Scholar] [CrossRef]
- Zhuang, Z.; Peng, Q.; Zhang, B.; Li, Y. Controllable Synthesis of Cu2S Nanocrystals and Their Assembly into a Superlattice. J. Am. Chem. Soc. 2008, 130, 10482–10483. [Google Scholar] [CrossRef]
- Xie, Y.; Riedinger, A.; Prato, M.; Casu, A.; Genovese, A.; Guardia, P.; Sottini, S.; Sangregorio, C.; Miszta, K.; Ghosh, S.; et al. Copper Sulfide Nanocrystals with Tunable Composition by Reduction of Covellite Nanocrystals with Cu+ Ions. J. Am. Chem. Soc. 2013, 135, 17630–17637. [Google Scholar] [CrossRef]
- Wu, Y.; Wadia, C.; Ma, W.; Sadtler, B.; Alivisatos, A.P. Synthesis and Photovoltaic Application of Copper(I) Sulfide Nanocrystals. Nano Lett. 2008, 8, 2551–2555. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, D.; Liang, J.; Shen, J.; Zhang, S.; Qian, Y. Growth of Cu2S Ultrathin Nanowires in a Binary Surfactant Solvent. J. Phys. Chem. B 2005, 109, 10699–10704. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Kruszynska, M.; Borchert, H.; Bachmatiuk, A.; Rümmeli, M.H.; Büchner, B.; Parisi, J.; Kolny-Olesiak, J. Size and Shape Control of Colloidal Copper(I) Sulfide Nanorods. ACS Nano 2012, 6, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.B.; Ghezelbash, A.; Hanrath, T.; Saunders, A.E.; Lee, F.; Korgel, B.A. Solventless Synthesis of Monodisperse Cu2S Nanorods, Nanodisks, and Nanoplatelets. J. Am. Chem. Soc. 2003, 125, 16050–16057. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Xu, W.; Li, X.; Guo, P.; Zhang, Y.; Liu, J. Cu2S nanosheets for ultrashort pulse generation in the near-infrared region. Nanoscale 2019, 11, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, H.; Niu, J.; Li, S.; Zhang, Y.; Wang, H.; Li, L.S. Columnar Self-Assembly of Cu2S Hexagonal Nanoplates Induced by Tin(IV)−X Complex as Inorganic Surface Ligand. J. Am. Chem. Soc. 2010, 132, 12778–12779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Zhang, Q.; Ge, J.; Lu, Z.; Hou, Y.; Yin, Y. One-Pot Synthesis and Optical Property of Copper(I) Sulfide Nanodisks. Inorg. Chem. 2010, 49, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Schulmeyer, T.; Brötz, J.; Klein, A.; Jaegermann, W. Interface properties and band alignment of Cu2S/CdS thin film solar cells. Thin Solid Films 2003, 431-432, 477–482. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, K.; Yang, G.; Li, Y.; Liu, X.; Chang, K.; Xuan, Y.; Ye, J. Ultrafine nano 1T-MoS2 monolayers with NiOx as dual co-catalysts over TiO2 photoharvester for efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 279, 119387. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, F.; Su, K.; Zhang, N.; Zhang, Y.; Wang, M.; Wang, X. Engineering Mo-O-C interface in MoS2@rGO via charge transfer boosts hydrogen evolution. Chem. Eng. J. 2020, 399, 126018. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Tian, J.; Sun, B.; Liang, Z.; Xu, X.; Cui, H. Controllable growth of MoS2 nanosheets on novel Cu2S snowflakes with high photocatalytic activity. Appl. Catal. B Environ. 2018, 232, 355–364. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Jiao, S. CuS@defect-rich MoS2 core-shell structure for enhanced hydrogen evolution. J. Colloid Interface Sci. 2020, 564, 77–87. [Google Scholar] [CrossRef]
- Ha, E.; Liu, W.; Wang, L.; Man, H.-W.; Hu, L.; Tsang, S.C.E.; Chan, C.T.-L.; Kwok, W.-M.; Lee, L.Y.S.; Wong, K.-Y. Cu2ZnSnS4/MoS2-Reduced Graphene Oxide Heterostructure: Nanoscale Interfacial Contact and Enhanced Photocatalytic Hydrogen Generation. Sci. Rep. 2017, 7, 39411. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef]
- Zhu, B.; Lin, B.; Zhou, Y.; Sun, P.; Yao, Q.; Chen, Y.; Gao, B. Enhanced photocatalytic H2 evolution on ZnS loaded with graphene and MoS2 nanosheets as cocatalysts. J. Mater. Chem. A 2014, 2, 3819–3827. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lian, J.; Cui, P.; Xu, Y.; Seo, S.; Lee, J.; Chan, Y.; Lee, H. Dual n-type doped reduced graphene oxide field effect transistors controlled by semiconductor nanocrystals. Chem. Commun. 2012, 48, 4052–4054. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, J.; Wang, L.; Wang, W.; Bian, J.; Feng, L.; Li, C. A novel Au NPs-loaded MoS2/RGO composite for efficient hydrogen evolution under visible light. Mater. Lett. 2016, 182, 125–128. [Google Scholar] [CrossRef]
- Wang, B.; An, W.; Liu, L.; Chen, W.; Liang, Y.; Cui, W. Novel Cu2S quantum dots coupled flower-like BiOBr for efficient photocatalytic hydrogen production under visible light. RSC Adv. 2015, 5, 3224–3231. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Long, R. Rapid Charge Separation Boosts Solar Hydrogen Generation at the Graphene–MoS2 Junction: Time-Domain Ab Initio Analysis. J. Phys. Chem. Lett. 2021, 12, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]





| Photocatalyst | Light Source | Sacrificial Reagent | H2 Production Rate (μmol g−1 h−1) | Reference |
|---|---|---|---|---|
| Cu2S-MoS2/rGO | 150 W Xenon | 0.35M Na2S/0.25M Na2SO3 | 324 | This work |
| Au/MoS2/rGO | 300 W Xenon | 0.25M methanol aq. | 115.4 | [29] |
| Cu2S/Pt | 300 W Xenon | 0.1M Na2S/0.5M Na2SO3 | 241.2 | [30] |
| TiO2/MoS2/rGO | 350 W Xenon | 25% (v/v) ethanol/water | 165.3 | [27] |
| Cu2ZnSnS4/MoS2/rGO | 150 W Xenon | 0.35M Na2S/0.25M Na2SO3 | 104 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, E.; Xin, Z.; Li, D.; Zhang, J.; Ji, T.; Hu, X.; Wang, L.; Hu, J. Dual-Modified Cu2S with MoS2 and Reduced Graphene Oxides as Efficient Photocatalysts for H2 Evolution Reaction. Catalysts 2021, 11, 1278. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111278
Ha E, Xin Z, Li D, Zhang J, Ji T, Hu X, Wang L, Hu J. Dual-Modified Cu2S with MoS2 and Reduced Graphene Oxides as Efficient Photocatalysts for H2 Evolution Reaction. Catalysts. 2021; 11(11):1278. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111278
Chicago/Turabian StyleHa, Enna, Zongyuan Xin, Danyang Li, Jingge Zhang, Tao Ji, Xin Hu, Luyang Wang, and Junqing Hu. 2021. "Dual-Modified Cu2S with MoS2 and Reduced Graphene Oxides as Efficient Photocatalysts for H2 Evolution Reaction" Catalysts 11, no. 11: 1278. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111278