Activation of Pt Nanoclusters on TiO2 via Tuning the Metallic Sites to Promote Low-Temperature CO Oxidation
Abstract
:1. Introduction
2. Results
2.1. Chemical and Structural Modifications
2.2. Surface Reduction Properties
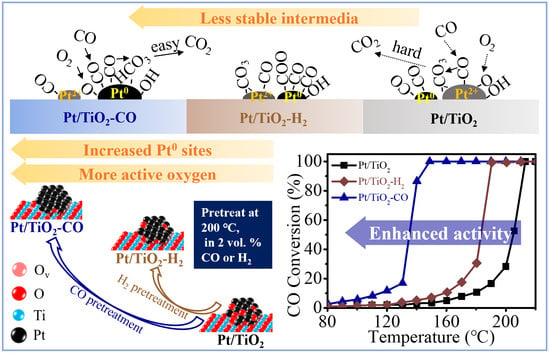
2.3. Catalytic Performance
2.4. In Situ DRIFTS Study
2.4.1. In Situ CO Adsorption–Desorption–Oxidation
2.4.2. In Situ CO Oxidation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalysts Characterization
4.4. Catalytic Performance Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nan, B.; Fu, Q.; Yu, J.; Shu, M.; Zhou, L.-L.; Li, J.; Wang, W.-W.; Jia, C.-J.; Ma, C.; Chen, J.-X.; et al. Unique structure of active platinum-bismuth site for oxidation of carbon monoxide. Nat. Commun. 2021, 12, 3342. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.-X.; Allard, L.F.; Lee, S.; Liu, J.; Li, H.; Wang, J.; Wang, J.; Oh, S.H.; Li, W.; et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. 2021, 28, 24847–24871. [Google Scholar] [CrossRef]
- Tripathi, A.; Hareesh, C.; Sinthika, S.; Andersson, G.; Thapa, R. CO oxidation on Pt based binary and ternary alloy nanocatalysts: Reaction pathways and electronic descriptor. Appl. Surf. Sci. 2020, 528, 146964. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Cuihua Xuebao/Chinese J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Newton, M.A.; Ferri, D.; Smolentsev, G.; Marchionni, V.; Nachtegaal, M. Room-temperature carbon monoxide oxidation by oxygen over Pt/Al2O3 mediated by reactive platinum carbonates. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Beniya, A.; Higashi, S.; Ohba, N.; Jinnouchi, R.; Hirata, H.; Watanabe, Y. CO oxidation activity of non-reducible oxide-supported mass-selected few-atom Pt single-clusters. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Guzman, J.; Carrettin, S.; Fierro-Gonzalez, J.C.; Hao, Y.; Gates, B.C.; Corma, A. CO oxidation catalyzed by supported gold: Cooperation between gold and nanocrystalline rare-earth supports forms reactive surface superoxide and peroxide species. Angew. Chem.-Int. Ed. 2005, 44, 4778–4781. [Google Scholar] [CrossRef]
- Gerrard, A.L.; Weaver, J.F. Kinetics of CO oxidation on high-concentration phases of atomic oxygen on Pt(111). J. Chem. Phys. 2005, 123, 224703. [Google Scholar] [CrossRef]
- Jan, A.; Shin, J.; Ahn, J.; Yang, S.; Yoon, K.J.; Son, J.-W.; Kim, H.; Lee, J.-H.; Ji, H.-I. Promotion of Pt/CeO2 catalyst by hydrogen treatment for low-temperature CO oxidation. RSC Adv. 2019, 9, 27002–27012. [Google Scholar] [CrossRef] [Green Version]
- DeRita, L.; Resasco, J.; Dai, S.; Boubnov, A.; Thang, H.V.; Hoffman, A.S.; Ro, I.; Graham, G.W.; Bare, S.R.; Pacchioni, G.; et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 2019, 18, 746–751. [Google Scholar] [CrossRef]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Influence of the pretreatment conditions on the development and performance of active sites of Pt/TiO2 catalysts used for the selective citral hydrogenation. J. Catal. 2015, 327, 86–95. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Vernoux, P.; Loridant, S.; Aires, F.J.C.S.; Epicier, T.; Betz, B.; Hoyer, R.; Grunwaldt, J.D. Tuning the Structure of Platinum Particles on Ceria In Situ for Enhancing the Catalytic Performance of Exhaust Gas Catalysts. Angew. Chem.-Int. Ed. 2017, 56, 13078–13082. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moemen, A.; Abdel-Mageed, A.M.; Bansmann, J.; Parlinska-Wojtan, M.; Behm, R.J.; Kučerová, G. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Oh, S.; Ha, H.; Choi, H.; Jo, C.; Cho, J.; Choi, H.; Ryoo, R.; Kim, H.Y.; Park, J.Y. Oxygen activation on the interface between Pt nanoparticles and mesoporous defective TiO2 during CO oxidation. J. Chem. Phys. 2019, 151, 234716. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Su, X.; Duan, H.; Liang, B.; Huang, Y.; Zhang, T. Catalytic performance of the Pt/TiO2 catalysts in reverse water gas shift reaction: Controlled product selectivity and a mechanism study. Catal. Today 2017, 281, 312–318. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Bañares, M.A.; Weng, L.-T.; Gu, Q.; Price, J.; Han, W.; Yeung, K.L. A Novel Approach to High-Performance Aliovalent-Substituted Catalysts—2D Bimetallic MOF-Derived CeCuOx Microsheets. Small 2019, 15, 1903525. [Google Scholar] [CrossRef]
- Kȩpiński, L.; Wołcyrz, M. Microstructure of Pd/CeO2 catalyst: Effect of high temperature reduction in hydrogen. Appl. Catal. A Gen. 1997, 150, 197–220. [Google Scholar] [CrossRef]
- Apopei, P.; Catrinescu, C.; Teodosiu, C.; Royer, S. Mixed-phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4-chlorophenol from aqueous effluents. Appl. Catal. B Environ. 2014, 160–161, 374–382. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Su, B.; Xu, J.; Ding, Z.; Wang, S. Unravelling the Promotional Effect of La2O3 in Pt/La-TiO2 Catalysts for CO2 Hydrogenation. Chem.-A Eur. J. 2020, 26, 517–523. [Google Scholar] [CrossRef]
- Macino, M.; Barnes, A.J.; Althahban, S.M.; Qu, R.; Gibson, E.K.; Morgan, D.J.; Freakley, S.J.; Dimitratos, N.; Kiely, C.J.; Gao, X.; et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat. Catal. 2019, 2, 873–881. [Google Scholar] [CrossRef]
- Motin, A.M.; Haunold, T.; Bukhtiyarov, A.V.; Bera, A.; Rameshan, C.; Rupprechter, G. Surface science approach to Pt/carbon model catalysts: XPS, STM and microreactor studies. Appl. Surf. Sci. 2018, 440, 680–687. [Google Scholar] [CrossRef]
- Li, N.; Chen, Q.-Y.; Luo, L.-F.; Huang, W.-X.; Luo, M.-F.; Hu, G.-S.; Lu, J.-Q. Kinetic study and the effect of particle size on low temperature CO oxidation over Pt/TiO2 catalysts. Appl. Catal. B Environ. 2013, 142–143, 523–532. [Google Scholar] [CrossRef]
- Benkoula, S.; Sublemontier, O.; Patanen, M.; Nicolas, C.; Sirotti, F.; Naitabdi, A.; Gaie-Levrel, F.; Antonsson, E.; Aureau, D.; Ouf, F.-X.; et al. Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: Bulk materials vs. isolated nanoparticles. Sci. Rep. 2015, 5, 15088. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, W.; Zhou, Z.; Huang, Q.; Liu, Y.; Duan, Q. Hydroxyl groups attached to Co2+ on the surface of Co3O4: A promising structure for propane catalytic oxidation. Catal. Sci. Technol. 2020, 10, 2573–2582. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, S.M.; Hong, S.C.; Kim, S.S. Active oxygen species adsorbed on the catalyst surface and its effect on formaldehyde oxidation over Pt/TiO2 catalysts at room temperature; Role of the Pt valence state on this reaction? RSC Adv. 2018, 8, 3626–3636. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Leung, D.Y.C. Complete elimination of indoor formaldehyde over supported Pt catalysts with extremely low Pt content at ambient temperature. J. Catal. 2011, 280, 60–67. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Wang, Y.; Jensen, J.O.; Zhang, W. Coarsening of carbon black supported Pt nanoparticles in hydrogen. Nanotechnology 2017, 28, 475710. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Du, X.; Zhu, Y.; Ren, S.; Li, J. Catalytic oxidation of chlorobenzene over Pd-TiO2 /Pd-Ce/TiO2 catalysts. Catalysts 2020, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Neumann, S.; Gutmann, T.; Buntkowsky, G.; Paul, S.; Thiele, G.; Sievers, H.; Bäumer, M.; Kunz, S. Insights into the reaction mechanism and particle size effects of CO oxidation over supported Pt nanoparticle catalysts. J. Catal. 2019, 377, 662–672. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, Y.; Binder, A.J.; Tang, W.; Wang, S.; Liu, J. (Jimmy); Liu, H.; Lu, X.; Wang, Y.; Ding, Y.; et al. Activating low-temperature diesel oxidation by single-atom Pt on TiO2 nanowire array. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; He, H.; Tanaka, K. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Ke, J.; Zhu, W.; Jiang, Y.; Si, R.; Wang, Y.J.; Li, S.C.; Jin, C.; Liu, H.; Song, W.G.; Yan, C.H.; et al. Strong Local Coordination Structure Effects on Subnanometer PtOx Clusters over CeO2 Nanowires Probed by Low-Temperature CO Oxidation. ACS Catal. 2015, 5, 5164–5173. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, G.J.; Lee, S.H.; Hwang, I.H.; Hong, S.C.; Kim, S.S. Catalytic Performance of Ce0.6Y0.4O2-Supported Platinum Catalyst for Low-Temperature Water-Gas Shift Reaction. ACS Omega 2018, 3, 3156–3163. [Google Scholar] [CrossRef] [Green Version]
- DeRita, L.; Dai, S.; Lopez-Zepeda, K.; Pham, N.; Graham, G.W.; Pan, X.; Christopher, P. Catalyst Architecture for Stable Single Atom Dispersion Enables Site-Specific Spectroscopic and Reactivity Measurements of CO Adsorbed to Pt Atoms, Oxidized Pt Clusters, and Metallic Pt Clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhao, Y.; Lee, S.; Yang, S.; Liu, J.; Giannakakis, G.; Li, M.; Ouyang, M.; Wang, D.; Sykes, E.C.H.; et al. High-loading single Pt atom sites [Pt-O(OH)x] catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 2020, 6, eaba3809. [Google Scholar] [CrossRef]
- Liu, A.; Liu, X.; Liu, L.; Pu, Y.; Guo, K.; Tan, W.; Gao, S.; Luo, Y.; Yu, S.; Si, R.; et al. Getting Insights into the Temperature-Specific Active Sites on Platinum Nanoparticles for CO Oxidation: A Combined in Situ Spectroscopic and ab Initio Density Functional Theory Study. ACS Catal. 2019, 9, 7759–7768. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Somasundaran, P.; Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl. Acad. Sci. USA 2018, 115, E9261–E9270. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Cao, T.; Gao, Y.; Li, D.; Xiong, F.; Huang, W. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 2016, 120, 21472–21485. [Google Scholar] [CrossRef]
- Li, J.-J.; Zhu, B.-L.; Wang, G.-C.; Liu, Z.-F.; Huang, W.-P.; Zhang, S.-M. Enhanced CO catalytic oxidation over an Au–Pt alloy supported on TiO2 nanotubes: Investigation of the hydroxyl and Au/Pt ratio influences. Catal. Sci. Technol. 2018, 8, 6109–6122. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt Particle Size and Valence State on the Performance of Pt/TiO2 Catalysts for CO Oxidation at Room Temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Haruta, M. Role of water in CO oxidation on gold catalysts. Catal. Lett. 2014, 144, 1475–1486. [Google Scholar] [CrossRef]







| Catalysts | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Pt0/(Pt0 + Pt2+) | Oads/(Olatt + Oads) | Ti3+/(Ti3+ + Ti4+) |
|---|---|---|---|---|---|---|
| Pt/TiO2 | 62.2 | 0.3 | 14.2 | 16.7% | 12.5% | 4.5% |
| Pt/TiO2-H2 | 65.8 | 0.3 | 13.6 | 23.7% | 16.5% | 3.8% |
| Pt/TiO2-CO | 65.0 | 0.3 | 14.9 | 46.9% | 17.0% | 4.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Wang, Q. Activation of Pt Nanoclusters on TiO2 via Tuning the Metallic Sites to Promote Low-Temperature CO Oxidation. Catalysts 2021, 11, 1280. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111280
He K, Wang Q. Activation of Pt Nanoclusters on TiO2 via Tuning the Metallic Sites to Promote Low-Temperature CO Oxidation. Catalysts. 2021; 11(11):1280. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111280
Chicago/Turabian StyleHe, Kailin, and Qingyue Wang. 2021. "Activation of Pt Nanoclusters on TiO2 via Tuning the Metallic Sites to Promote Low-Temperature CO Oxidation" Catalysts 11, no. 11: 1280. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111280