Au-Ag/TiO2 Thin Films Preparation by Laser Ablation and Sputtering Plasmas for Its Potential Use as Photoanodes in Electrochemical Advanced Oxidation Processes (EAOP)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructural Characterization
2.2. Optical Characterization
2.3. Photoelectrocatalytic Experiments
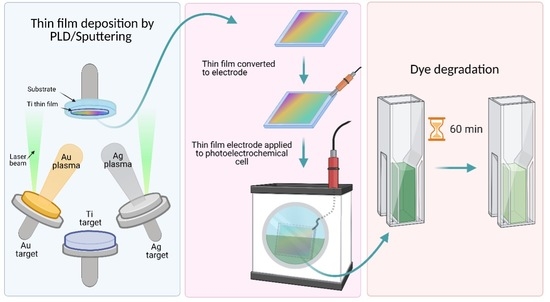
3. Materials and Methods
3.1. Thin Films Preparation
3.2. Thin Films Characterization
3.3. Photoanodes Preparation and Photoelectrocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Alarcon, L.; Solís-Casados, D.A.; González-Zavala, F.; Romero, S.; Fernandez, M.; Haro-Poniatowski, E. Preparation of Nanostructured Bi-Modified TiO2 Thin Films by Crossed-Beam Laser Ablation Plasmas. In Proceedings of the Journal of Physics: Conference Series; Institute of Physics Publishing, IOP publisher: London, UK, 2017; Volume 792, p. 012006. [Google Scholar]
- Escobar-Alarcón, L.; Gonzalez-Zavala, F.; Solis-Casados, D.A.; Fernandez, M.; Aspiazu, J.; Haro-Poniatowski, E. Zn-Modified TiO2 Thin Films Deposited by Combining Plasmas Produced by Laser Ablation and Magnetron Sputtering. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–7. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Solis-Casados, D.A.; Romero, S.; Haro-Poniatowski, E. Thin Films Prepared by a Hybrid Deposition Configuration Combining Two Laser Ablation Plasmas with One Sputtering Plasma. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–8. [Google Scholar] [CrossRef]
- De Santiago Colín, D.M.; Martínez-Chávez, L.A.; Cuán, Á.; Elizalde-Peña, E.A.; Rivera, J.A.; Guzmán, C.; Escobar-Alarcón, L.; Esquivel, K. Sonochemical Coupled Synthesis of Cr-TiO2 supported on Fe3O4 structures and Chemical Simulation of the Degradation Mechanism of Malachite Green Dye. J. Photochem. Photobiol. A Chem. 2018, 364. [Google Scholar] [CrossRef]
- Silva, T.A.; Diniz, J.; Paixão, L.; Vieira, B.; Barrocas, B.; Nunes, C.D.; Monteiro, O.C. Novel Titanate Nanotubes-Cyanocobalamin Materials: Synthesis and Enhanced Photocatalytic Properties for Pollutants Removal. Solid State Sci. 2017, 63, 30–41. [Google Scholar] [CrossRef]
- López, R.; Gómez, R.; Oros-Ruiz, S. Photophysical and Photocatalytic Properties of TiO2-Cr Sol–Gel Prepared Semiconductors. Catal. Today 2011, 166, 159–165. [Google Scholar] [CrossRef]
- Fang, J.; Cao, S.-W.; Wang, Z.; Shahjamali, M.M.; Loo, S.C.J.; Barber, J.; Xue, C. Mesoporous Plasmonic Au–TiO2 Nanocomposites for Efficient Visible-Light-Driven Photocatalytic Water Reduction. Int. J. Hydrog. Energy 2012, 37, 17853–17861. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2-Based Photocatalysts: A Review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ishii, Y.; Yamane, H.; Watanabe, K.; Koda, H.; Kunigami, H.; Kunigami, H. Fabrication of TiO2/Pt Core–Shell Particles by Electroless Metal Plating. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 448, 88–92. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and Characterization of Ag-Doped TiO2 Nanomaterials and Their Photocatalytic Reduction of Cr(VI) under Visible Light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Cybula, A.; Nowaczyk, G.; Jarek, M.; Zaleska, A. Preparation and Characterization of Au/Pd Modified-TiO2 Photocatalysts for Phenol and Toluene Degradation under Visible Light—The Effect of Calcination Temperature. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of Metal-Doping of TiO2 Nanoparticles on Their Photocatalytic Activities toward Removal of Organic Dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Li, F.B.; Li, X.Z. The Enhancement of Photodegradation Efficiency Using Pt–TiO2 Catalyst. Chemosphere 2002, 48, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Segura, S.; Brillas, E. Applied Photoelectrocatalysis on the Degradation of Organic Pollutants in Wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Gerlach, J.W.; Mändl, S. Correlation between RBS, Reflectometry and Ellipsometry Data for TiO2 Films Deposited on Si. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 242, 289–292. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Y.; Zhao, X. Combination of Photocatalytic and Electrochemical Degradation of Organic Pollutants from Water. Curr. Opin. Green Sustain. Chem. 2017, 6, 78–84. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Song, H.; Sun, T.; Wan, J. Preparation of RuO2-TiO2/Nano-Graphite Composite Anode for Electrochemical Degradation of Ceftriaxone Sodium. J. Hazard. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Zhang, M.; Jiang, X.; Wang, Y.; Lv, J.; He, G.; Sun, Z. Three-Dimensional Hierarchical Anatase@rutile TiO2 Nanotree Array Films Decorated by Silver Nanoparticles as Ultrasensitive Recyclable Surface-Enhanced Raman Scattering Substrates. J. Alloys Compd. 2017, 725, 1166–1174. [Google Scholar] [CrossRef]
- Nechache, R.; Nicklaus, M.; Diffalah, N.; Ruediger, A.; Rosei, F. Pulsed Laser Deposition Growth of Rutile TiO2 Nanowires on Silicon Substrates. Appl. Surf. Sci. 2014, 313, 48–52. [Google Scholar] [CrossRef]
- Yang, K.-H.; Chang, C.-M. Surface-Enhanced Raman Scattering-Active Au/TiO2 Films Prepared by Electrochemical and Photochemical Methods. Mater. Res. Bull. 2013, 48, 372–377. [Google Scholar] [CrossRef]
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2/Au/TiO2 Multilayer Thin-Film Photoanodes Synthesized by Pulsed Laser Deposition for Photoelectrochemical Degradation of Organic Pollutants. Sep. Purif. Technol. 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Graillot-Vuillecot, R.; Thomann, A.L.; Lecas, T.; Cachoncinlle, C.; Millon, E.; Caillard, A. Hot Target Magnetron Sputtering Process: Effect of Infrared Radiation on the Deposition of Titanium and Titanium Oxide Thin Films. Vacuum 2020, 181, 109734. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.H.; Han, J.G. Reactive Magnetron Sputtering of Thin Films: Present Status and Trends. In Thin Solid Films; Elsevier: Amsterdam, The Netherlands, 2005; Volume 475, pp. 208–218. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed Laser Ablation and Deposition of Thin Films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of Making TiO2 and ZnO Visible Light Active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Mädler, L.; Amal, R. Inter-Relationship between Pt Oxidation States on TiO2 and the Photocatalytic Mineralisation of Organic Matters. J. Catal. 2007, 251, 271–280. [Google Scholar] [CrossRef]
- Krzanowski, J.E.; Endrino, J.L.; Nainaparampil, J.J.; Zabinski, J.S. Composite Coatings Incorporating Solid Lubricant Phases. In Proceedings of the Journal of Materials Engineering and Performance; Springer: Berlin/Heidelberg, Germany, 2004; Volume 13, pp. 439–444. [Google Scholar]
- Martínez-Chávez, L.A.; Esquivel, K.; Solis-Casados, D.A.; Velázquez-Castillo, R.; Haro-Poniatowski, E.; Escobar-Alarcón, L. Nanocomposite Bi/TiO2 Multilayer Thin Films Deposited by a Crossed Beam Laser Ablation Configuration. Appl. Phys. A 2021, 127, 1–9. [Google Scholar] [CrossRef]
- González-Zavala, F.; Escobar-Alarcón, L.; Solís-Casados, D.A.; Rebollar, D.A.; Basurto, R.; Haro-Poniatowski, E. Deposition and Photocatalytic Activity of Ag: V2O5 Thin Films. In Proceedings of the Materials Research Society Symposium Proceedings; Materials Research Society, Cancún, México, 1 June 2016; Volume 1817, pp. 22–27. [Google Scholar]
- Gonzalez-Zavala, F.; Escobar-Alarcón, L.; Solís-Casados, D.A.; Rivera-Rodríguez, C.; Basurto, R.; Haro-Poniatowski, E. Preparation of Vanadium Oxide Thin Films Modified with Ag Using a Hybrid Deposition Configuration. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–6. [Google Scholar] [CrossRef]
- Hernández, R.; Hernández-Reséndiz, J.R.; Cruz-Ramírez, M.; Velázquez-Castillo, R.; Escobar-Alarcón, L.; Ortiz-Frade, L.; Esquivel, K. Au-TiO2 Synthesized by a Microwave- and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst. Catalysts 2020, 10, 1052. [Google Scholar] [CrossRef]
- Kimura, K.; Naya, S.; Jin-nouchi, Y.; Tada, H. TiO2 Crystal Form-Dependence of the Au/TiO2 Plasmon Photocatalyst’s Activity. J. Phys. Chem. C 2012, 116, 7111–7117. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, D.J.; Ahn, H. XRD and TEM Studies on Tin Oxide (II) Nanoparticles Prepared by Inert Gas Condensation. Mater. Lett. 2004, 58, 3122–3125. [Google Scholar] [CrossRef]
- Scipioni, R.; Gazzoli, D.; Teocoli, F.; Palumbo, O.; Paolone, A.; Ibris, N.; Brutti, S.; Navarra, M.A. Preparation and Characterization of Nanocomposite Polymer Membranes Containing Functionalized SnO2 Additives. Membranes 2014, 4, 123–142. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Qiao, X.; Luo, F.; Wan, B.; Zhang, C. Low-Cost High- Performance NO2 Sensor Based on Nanoporous Indium Tin Oxide (ITO) Film. Sensors Actuators B Chem. 2021, 346, 130440. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Titanium Dioxide (TiO2) Nanoparticles-XRD Analyses-An Insight; arXivLabs; Cornell University: Ithaca, NY, USA, 2013. [Google Scholar]
- Rodrigues, M.S.; Borges, J.; Gabor, C.; Munteanu, D.; Apreutesei, M.; Steyer, P.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; et al. Functional Behaviour of TiO2 Films Doped with Noble Metals. Surf. Eng. 2016, 32, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall Analysis in Estimation of Lattice Strain in Nanometer-Sized ZnO Particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Khorsand Zak, A.; Abd Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–Strain Plot Methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Jing, Z.; Meijun, L.; Zhaochi, F.; Jun, C.; Li, C. UV Raman Spectroscopic Study on TiOI. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Toxqui-Teran, A.; Vega-Becerra, O.; Miki-Yoshida, M.; Rojas-Villalobos, M.; García-Guaderrama, M.; Aguilar-Martínez, J.A. Low-Temperature Synthesis and Characterization of Anatase TiO2 Nanoparticles by an Acid Assisted Sol–Gel Method. J. Alloys Compd. 2015, 647, 627–636. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.Y.; Yeo, I.S.; Kim, T.U.; Ki, H.C.; Gu, H.B. Surface Plasmon Resonance Effect of Silver Nanoparticles on a TiO2 Electrode for Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2018, 432, 266–271. [Google Scholar] [CrossRef]
- Wisniewska, J.; Guesmi, H.; Ziolek, M.; Tielens, F. Stability of Nanostructured Silver-Platinum Alloys. J. Alloys Compd. 2019, 770, 934–941. [Google Scholar] [CrossRef]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Naumov, V.; Fedorenko, L.; Kshnyakin, V.; Baran, J. Room Temperature Photoluminescence of Anatase and Rutile TiO2 Powders. J. Lumin. 2014, 146, 199–204. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of Anatase and Rutile TiO2 Particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef]
- Chen, C.C.; Lu, C.S.; Chung, Y.C.; Jan, J.L. UV Light Induced Photodegradation of Malachite Green on TiO2 Nanoparticles. J. Hazard. Mater. 2007, 141, 520–528. [Google Scholar] [CrossRef]
- Ju, Y.; Qiao, J.; Peng, X.; Xu, Z.; Fang, J.; Yang, S.; Sun, C. Photodegradation of Malachite Green Using UV-Vis Light from Two Microwave-Powered Electrodeless Discharge Lamps (MPEDL-2): Further Investigation on Products, Dominant Routes and Mechanism. Chem. Eng. J. 2013, 221, 353–362. [Google Scholar] [CrossRef]
- Liu, X.; An, S.; Shi, W.; Yang, Q.; Zhang, L. Microwave-Induced Catalytic Oxidation of Malachite Green under Magnetic Cu-Ferrites: New Insight into the Degradation Mechanism and Pathway. J. Mol. Catal. A Chem. 2014, 395, 243–250. [Google Scholar] [CrossRef]
- Qin, J.; Ye, S.; Yan, K.; Zhang, J. Visible Light-Driven Photoelectrocatalysis for Simultaneous Removal of Oxytetracycline and Cu (II) Based on Plasmonic Bi/Bi2O3/TiO2 Nanotubes. J. Colloid Interface Sci. 2022, 607, 1936–1943. [Google Scholar] [CrossRef]
- Matarrese, R.; Mascia, M.; Vacca, A.; Mais, L.; Usai, E.; Ghidelli, M.; Mascaretti, L.; Bricchi, B.; Russo, V.; Casari, C.; et al. Integrated Au/TiO2 Nanostructured Photoanodes for Photoelectrochemical Organics Degradation. Catalysts 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Orimolade, B.O.; Zwane, B.N.; Koiki, B.A.; Tshwenya, L.; Peleyeju, G.M.; Mabuba, N.; Zhou, M.; Arotiba, O.A. Solar Photoelectrocatalytic Degradation of Ciprofloxacin at a FTO/BiVO4/MnO2 Anode: Kinetics, Intermediate Products and Degradation Pathway Studies. J. Environ. Chem. Eng. 2020, 8, 103607. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Fulgione, I.; Frontistis, Z.; Rizzo, L.; Mantzavinos, D. Solar Light-Induced Photoelectrocatalytic Degradation of Bisphenol-A on TiO2/ITO Film Anode and BDD Cathode. Catal. Today 2013, 209, 74–78. [Google Scholar] [CrossRef]
- Solís-Casados, D.A.; Escobar-Alarcón, L.; Fernández, M.; Valencia, F. Malachite Green Degradation in Simulated Wastewater Using Nix:TiO2 Thin Films. Fuel 2013, 110, 17–22. [Google Scholar] [CrossRef]
- Mohite, V.S.; Mahadik, M.A.; Kumbhar, S.S.; Hunge, Y.M.; Kim, J.H.; Moholkar, A.V.; Rajpure, K.Y.; Bhosale, C.H. Photoelectrocatalytic Degradation of Benzoic Acid Using Au Doped TiO2 Thin Films. J. Photochem. Photobiol. B Biol. 2015, 142, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Reddy, I.N.; Kwon, J.H.; Yi, J.; Sohn, Y.; Gwag, J.S.; Noh, J.S. Charge Carrier Generation and Control on Plasmonic Au Clusters Functionalized TiO2 Thin Films for Enhanced Visible Light Water Splitting Activity. Ceram. Int. 2018, 44, 18978–18986. [Google Scholar] [CrossRef]
- Landolsi, Z.; Ben Assaker, I.; Nunes, D.; Fortunato, E.; Martins, R.; Chtourou, R.; Ammar, S. Enhanced Electrical and Photocatalytic Properties of Porous TiO2 Thin Films Decorated with Fe2O3 Nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 20753–20773. [Google Scholar] [CrossRef]
- Díaz-Real, J.A.; Ma, J.; Alonso-Vante, N. Highly Photoactive Brookite and Anatase with Enhanced Photocatalytic Activity for the Degradation of Indigo Carmine Application. Appl. Catal. B Environ. 2016, 198, 471–479. [Google Scholar] [CrossRef]














| Sample | KObs (min−1) | Sample | KObs (min−1) |
|---|---|---|---|
| TiO2-Ag (EC) | 0.00987 | TiO2-Ag (PEC) | 0.025 |
| TiO2-Au (EC) | 0.01756 | TiO2-Au (PEC) | 0.02867 |
| TiO2-Ag-Au (EC) | 0.02124 | TiO2-Ag-Au (PEC) | 0.02595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Chávez, L.A.; Rivera-Muñoz, E.M.; Velázquez-Castillo, R.R.; Escobar-Alarcón, L.; Esquivel, K. Au-Ag/TiO2 Thin Films Preparation by Laser Ablation and Sputtering Plasmas for Its Potential Use as Photoanodes in Electrochemical Advanced Oxidation Processes (EAOP). Catalysts 2021, 11, 1406. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111406
Martínez-Chávez LA, Rivera-Muñoz EM, Velázquez-Castillo RR, Escobar-Alarcón L, Esquivel K. Au-Ag/TiO2 Thin Films Preparation by Laser Ablation and Sputtering Plasmas for Its Potential Use as Photoanodes in Electrochemical Advanced Oxidation Processes (EAOP). Catalysts. 2021; 11(11):1406. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111406
Chicago/Turabian StyleMartínez-Chávez, Luis Alejandro, Eric Mauricio Rivera-Muñoz, Rodrigo Rafael Velázquez-Castillo, Luis Escobar-Alarcón, and Karen Esquivel. 2021. "Au-Ag/TiO2 Thin Films Preparation by Laser Ablation and Sputtering Plasmas for Its Potential Use as Photoanodes in Electrochemical Advanced Oxidation Processes (EAOP)" Catalysts 11, no. 11: 1406. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111406