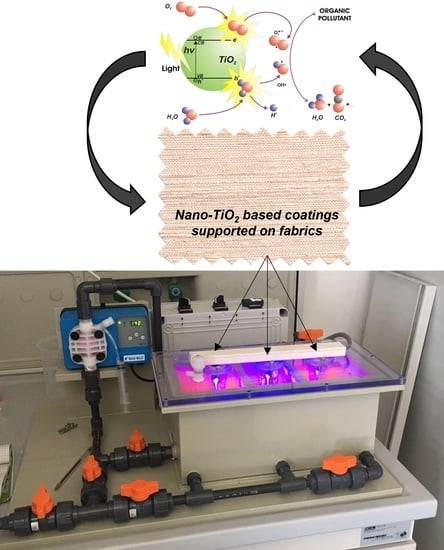
Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of TiO2-Based Nanosuspension
2.2. Characterization of Fabrics
2.3. Optimization of Photocatalytic Process
2.3.1. Effect of TiO2-Based Coatings Composition
2.3.2. Effect of Temperature
2.3.3. Effect of Fabric Substrate
2.4. Process Scalability
3. Materials and Methods
3.1. Materials
3.2. TiO2-Based Nanosuspensions
3.3. Ceramized Fabric
3.4. Semi-Pilot Plant and Irradiation Source
3.5. Characterization
3.5.1. Dynamic Light Scattering/Electrophoretic Light Scattering
3.5.2. Characterization on Fabrics
3.6. Rhodamine B Degradation Tests
- where
- mol of product is calculated as the initial concentration of reagent per efficiency reached at the time s
- mol of catalyst are the moles of the catalyst deposited on the exposure area of fabric calculated by the AO% parameter.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organizzazione delle Nazioni Unite. Trasformare il Nostro Mondo: L’Agenda 2030 per lo Sviluppo Sostenibile (Agenda2030). In Risoluzione Adottata Dall’assemblea Gen. 25 Settembre 2015; 2015; Available online: https://unric.org/it/wp-content/uploads/sites/3/2019/11/Agenda-2030-Onu-italia.pdf.1 (accessed on 1 November 2021).
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Varenne, F.; Hillaireau, H.; Bataille, J.; Smadja, C.; Barratt, G.; Vauthier, C. Application of validated protocols to characterize size and zeta potential of dispersed materials using light scattering methods. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 418–425. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.T.; Li, X.Z.; Li, W.Z.; Wu, M.; Bao, X.H. The enhancement of TiO2 photocatalytic activity by hydrogen thermal treatment. Chemosphere 2003, 50, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Yuranova, T.; Laub, D.; Kiwi, J. Synthesis, activity and characterization of textiles showing self-cleaning activity under daylight irradiation. Catal. Today 2007, 122, 109–117. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Palmisano, L.; Parrino, F.; Buscarino, G.; Amadelli, R. Preparation and photoactivity of samarium loaded anatase, brookite and rutile catalysts. Appl. Catal. B Environ. 2011, 104, 291–299. [Google Scholar] [CrossRef]
- Adly, M.S.; El-Dafrawy, S.M.; El-Hakam, S.A. Application of nanostructured graphene oxide/titanium dioxide composites for photocatalytic degradation of rhodamine B and acid green 25 dyes. J. Mater. Res. Technol. 2019, 8, 5610–5622. [Google Scholar] [CrossRef]
- Khodadadian, F.; de Boer, M.W.; Poursaeidesfahani, A.; van Ommen, J.R.; Stankiewicz, A.I.; Lakerveld, R. Design, characterization and model validation of a LED-based photocatalytic reactor for gas phase applications. Chem. Eng. J. 2018, 333, 456–466. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment—A review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Costa, A.L.; Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M. TiO2 based photocatalytic coatings: From nanostructure to functional properties. Chem. Eng. J. 2013, 225, 880–886. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Suresh Deshpande, A. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2021, 576, 151745. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Dong, X.; Xu, J.; Kong, C.; Zeng, X.; Wang, J.; Zhao, Y.; Zhang, W. Synthesis of β-FeOOH/TiO2/SiO2 by melting phase separation-hydrothermal method to improve photocatalytic performance. Ceram. Int. 2021, 47, 32303–32309. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Blosi, M.; Zanoni, I.; Costa, A.L. NanoTiO2 @DNA complex: A novel eco, durable, fire retardant design strategy for cotton textiles. J. Colloid Interface Sci. 2019, 546, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Factories, H.; Study, A.C.; Plant, P.; Joseane, J.; Mesa, M.; Sebasti, J.; Gonz, W.; Rojas, H.; Murcia Mesa, J.J.; Hernández Niño, J.S.; et al. Photocatalytic Treatment of Stained Wastewater Coming from Handicraft Factories. A Case Study at the Pilot Plant Level. Water 2021, 13, 2705. [Google Scholar]
- Sciscenko, I.; Mestre, S.; Climent, J.; Valero, F.; Escudero-Oñate, C.; Oller, I.; Arques, A. Magnetic photocatalyst for wastewater tertiary treatment at pilot plant scale: Disinfection and enrofloxacin abatement. Water 2021, 13, 329. [Google Scholar] [CrossRef]
- Qian, W.; Zhaoqun, W.; Xuanfeng, K.; Xiaodan, G.; Gi, X. A facile strategy for controlling the self-assembly of nanocomposite particles based on colloidal steric stabilization theory. Langmuir 2008, 24, 7778–7784. [Google Scholar] [CrossRef]
- Ortelli, S.; Poland, C.A.; Baldi, G.; Costa, A.L. Silica matrix encapsulation as a strategy to control ROS production while preserving photoreactivity in nano-TiO2. Environ. Sci. Nano 2016, 3, 602–610. [Google Scholar] [CrossRef]
- Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M.; Costa, A.L. TiO2 based nano-photocatalysis immobilized on cellulose substrates. J. Photochem. Photobiol. A Chem. 2014, 276, 58–64. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of Rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Xia, S.; Pan, G.; Xue, J.; Jiang, J.; Ni, Z. Preparation and photocatalytic activity of composite metal oxides derived from Salen-Cu(II) intercalated layered double hydroxides. Korean J. Chem. Eng. 2017, 34, 2331–2341. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Wang, J.; Tan, X.; Sun, H.; Liu, S.; Wang, S. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism. J. Colloid Interface Sci. 2018, 532, 321–330. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kanjwal, M.A.; Chronakis, I.S.; Kim, H.Y. Influence of temperature on the photodegradation process using Ag-doped TiO2 nanostructures: Negative impact with the nanofibers. J. Mol. Catal. A Chem. 2013, 366, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Hsu, Y.H. Effects of reaction temperature on the photocatalytic activity of TiO2 with Pd and Cu cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Parkin, I.P.; Nakata, K. Charge Carrier Transfer in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, ISBN 9780081028902. [Google Scholar]
- Alisawi, H.A.O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Ortelli, S.; Costa, A.L.; Dondi, M. TiO2 nanosols applied directly on textiles using different purification treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L.; Torri, C.; Samorì, C.; Galletti, P.; Vinais, C.; Varesano, A.; Bonura, L.; Bianchi, G. Innovative and sustainable production of Biopolymers. In Factories of the Future: The Italian Flagship Initiative; Tolio, T., Copani, G., Terkaj, W., Eds.; Springer International Publishing: Cham, Swizerland, 2019; Chapter 6; ISBN 9783319943589. [Google Scholar]






| Sample | dDLS (nm) | Z Potential (mV) | pH | pHi.e.,p. |
|---|---|---|---|---|
| TAC | 27 | +36 | 1.5 | 7.7 |
| TACR | 29 | +45 | 4 | 5.2 |
| SiO2 | 30 | −45 | 7 | <1.5 |
| SiO2-R | 37 | −34 | 4 | <1.5 |
| TiO2:SiO2 | 317 | +38 | 4 | 5.2 |
| Fabric | AO% (TACR) | AO% (TiO2:SiO2) |
|---|---|---|
| SP | 5.9 | 8 |
| SC | 8.4 | n.a. |
| SM | 6.2 | n.a. |
| C | 3.8 | 3 |
| Code | Images | Composition | g/m2 |
|---|---|---|---|
| SP |  | 65% cotton 35% polyester | 450 |
| SC |  | 65% cotton 35% polyester | 500 |
| SM |  | Not available | 640 |
| C |  | 100% cotton | 100 |
| Fabric | Untreated | Coated |
|---|---|---|
| SP | 121 ± 1 | 121 ± 3 |
| SC | 113 ± 1 | n.d. |
| SM | n.d. | n.d. |
| C | n.d. | 122 ± 4 |
| Irradiation Light | Coating | Photocatalytic Efficiency % |
|---|---|---|
| Visible | TACR | 49 |
| TiO2:SiO2 | 51 | |
| UV | TACR | 64 |
| TiO2:SiO2 | 60 |
| Irradiation | Temperature °C | Photocatalytic Efficiency % |
|---|---|---|
| Visible | 15 | 55 |
| 25 | 51 | |
| 38 | 57 | |
| UV | 15 | 63 |
| 25 | 60 | |
| 38 | 59 |
| Fabric | Photocatalytic Efficiency % | |
|---|---|---|
| UV LED | Visible LED | |
| SP | 49 | 64 |
| SC | 64 | 54 |
| SM | 65 | 61 |
| C | 67 | 57 |
| Fabric | Photocatalytic Efficiency % | |
|---|---|---|
| UV LED | Visible LED | |
| SP | 7.5 × 10−5 | 9.8 × 10−5 |
| SC | 8.9 × 10−5 | 7.5 × 10−5 |
| SM | 8.6 × 10−5 | 8.8 × 10−5 |
| C | 1.02 × 10−3 | 8.7 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccani, L.; Ortelli, S.; Blosi, M.; Costa, A.L. Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants. Catalysts 2021, 11, 1418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111418
Faccani L, Ortelli S, Blosi M, Costa AL. Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants. Catalysts. 2021; 11(11):1418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111418
Chicago/Turabian StyleFaccani, Lara, Simona Ortelli, Magda Blosi, and Anna Luisa Costa. 2021. "Ceramized Fabrics and Their Integration in a Semi-Pilot Plant for the Photodegradation of Water Pollutants" Catalysts 11, no. 11: 1418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111418