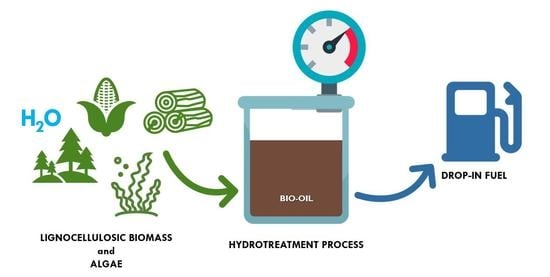
Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil
Abstract
:1. Introduction
2. Bio-Oil Proprieties
3. Catalytic Hydrogenation of BIO-Oil
3.1. Catalysts
3.2. Kinetic Mechanism
3.2.1. Phenol
3.2.2. Guaiacol
3.2.3. Levoglucosan
3.2.4. Other Compounds
3.3. Reactor Technologies
3.3.1. Conventional Reactors
3.3.2. Membrane Reactor
4. Concluding Remarks and Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BNZ | Benzene |
| CHE | Cyclohexene |
| CHO | Cyclohexanol |
| CMR | Catalytic membrane reactor |
| COL | Cyclohexanone |
| CXO | Cyclohexanol |
| Ea | Activation energy |
| EBR | Ebullated bed reactor |
| EG | Ethylene glycol |
| γ-BCT | γ-Butyrolactone |
| GCL | Guaiacol |
| GCS | Glucose |
| HDO | Hydrodeoxygenation reaction |
| HD | Hydrotreating |
| HTL | Hydrothermal liquefaction |
| HXD | Hydroxyacetaldehyde |
| HXE | Hydroxyacetone |
| k | Kinetic rate |
| k0 | Pre-exponential number |
| LG | Levoglucosan |
| LHHW | Langmuir–Hinshelwood–Hougen–Watson |
| LHSV | Liquid hourly space velocity |
| MR | Membrane reactor |
| MBR | Moving bed reactor |
| MCP | Methylcyclopentane |
| n | Kinetic order |
| PBR | Packet bed reactor |
| PBMR | Packed bed membrane reactor |
| PCL | Pyrocatechol |
| PHE | Phenol |
| SOB | Sorbitol |
| TEA | Techno-economical assessment |
| TOF | Turnover of frequency |
| WBO | Water soluble bio-oil fraction |
| 1,2-PDO | 1,2-Propanediol |
| 1,4-BDO | 1,4-Butanediol |
| 2-FN | 2-Furanone |
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Paone, E.; Beneduci, A.; Corrente, G.A.; Malara, A.; Mauriello, F. Hydrogenolysis of aromatic ethers under lignin-first conditions. Mol. Catal. 2020, 497, 111228. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Frontera, P.; Bonaccorsi, L.; Panzera, G.; Mauriello, F. Sustainable exploitation of coffee silverskin in water remediation. Sustainability 2020, 10, 3547. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. ChemSocRev 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 11 January 2021).
- Carlos, R.M.; Khang, D.B. Characterization of biomass energy projects in Southeast Asia. Biomass Bioenergy 2008, 32, 525–532. [Google Scholar] [CrossRef]
- Paone, E.; Espro, C.; Pietropaolo, R.; Mauriello, F. Selective arene production from transfer hydrogenolysis of benzyl phenyl ether promoted by a co-precipitated Pd/Fe3O4 catalyst. Catal. Sci. Technol. 2016, 6, 7937–7941. [Google Scholar] [CrossRef]
- Mauriello, F.; Paone, E.; Pietropaolo, R.; Balu, A.M.; Luque, R. Catalytic transfer hydrogenolysis of lignin-derived aromatic ethers promoted by bimetallic Pd/Ni systems. ACS Sustain. Chem. Eng. 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading lignocellulosic biomasses: Hydrogenolysis of platform derived molecules promoted by heterogeneous Pd-Fe catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic transfer hydrogenolysis as an effective tool for the reductive upgrading of cellulose, hemicellulose, lignin, and their derived molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Mauriello, F.; Ariga-Miwa, H.; Paone, E.; Pietropaolo, R.; Takakusagi, S.; Asakura, K. Transfer hydrogenolysis of aromatic ethers promoted by the bimetallic Pd/Co catalyst. Catal. Today 2020, 357, 511–517. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Bonaccorsi, L.; Mauriello, F.; Macario, A.; Frontera, P. Pd/Fe3O4 Nanofibers for the Catalytic Conversion of Lignin-Derived Benzyl Phenyl Ether under Transfer Hydrogenolysis Conditions. Catalysts 2020, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Srifa, A.; Chaiwat, W.; Pitakjakpipop, P.; Anutrasakda, W.; Faungnawakij, K. Chapter 6—Advances in bio-oil production and upgrading technologies. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–198. [Google Scholar]
- Han, Y.; Gholizadeh, M.; Tran, C.-C.; Kaliaguine, S.; Li, C.-Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Town, G. Review of solar energy for biofuel extraction. Renew. Sustain. Energy Rev. 2018, 88, 184–192. [Google Scholar] [CrossRef]
- Pistone, A.; Espro, C. Current trends on turning biomass wastes into carbon materials for electrochemical sensing and rechargeable battery applications. Curr. Opin. Green Sustain. Chem. 2020, 26, 100374. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Bagnato, G.; Boulet, F.; Sanna, A. Effect of Li-LSX zeolite, NiCe/Al2O3 and NiCe/ZrO2 on the production of drop-in bio-fuels by pyrolysis and hydrotreating of Nannochloropsis and isochrysis microalgae. Energy 2019, 179, 199–213. [Google Scholar] [CrossRef]
- Kumar, R.; Strezova, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Baker, E.G.; Elliott, D.C. Catalytic Hydrotreating of Biomass-Derived Oils, in Pyrolysis Oils from Biomass. ACS Symp. Ser. 1988, 21, 228–240. [Google Scholar]
- Bagnato, G.S.; Sanna, A. Process and Techno-Economic Analysis for Fuel and Chemical Production by Hydrodeoxygenation of Bio-Oil. Catalysts 2019, 9, 1021. [Google Scholar] [CrossRef] [Green Version]
- Lima, S.; Fernandes, A.; Antunes, M.M.; Pillinger, M.; Ribeiro, F.; Valente, A.A. Dehydration of Xylose into Furfural in the Presence of Crystalline Microporous Silicoaluminophosphates. Catal. Lett. 2010, 135, 41–47. [Google Scholar] [CrossRef]
- Demirbas, A. Thermochemical Processes. In Biorefineries: For Biomass Upgrading Facilities. Green Energy and Technology; Springer: London, UK, 2010; pp. 135–192. [Google Scholar]
- BTG Bioliquids BV. 2020. Available online: https://www.btg-bioliquids.com (accessed on 11 January 2021).
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Fisk, C.A.; Morgan, T.; Ji, Y.; Crocker, M.; Crofcheck, C.; Lewis, S.A. Bio-oil upgrading over platinum catalysts using in situ generated hydrogen. Appl. Catal. A Gen. 2009, 358, 150–156. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Wang, X.; Shao, J.; Lin, G.; Yang, H.; Yang, Q.; Chen, H. Catalytic Upgrading of Fast Pyrolysis Products with Fe-, Zr-, and Co-Modified Zeolites Based on Pyrolyzer–GC/MS Analysis. Energy Fuels 2017, 31, 3979–3986. [Google Scholar] [CrossRef]
- Dong, T.; Wang, J.; Miao, C.; Zheng, Y.; Chen, S. Two-step in situ biodiesel production from microalgae with high free fatty acid content. Bioresour. Technol. 2013, 136, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zainan, N.H.; Srivatsa, S.C.; Bhattacharya, S. Catalytic pyrolysis of microalgae Tetraselmis suecica and characterization study using in situ Synchrotron-based Infrared Microscopy. Fuel 2015, 161, 345–354. [Google Scholar] [CrossRef]
- Demirbas, A. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process. Technol. 2007, 88, 591–597. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]
- Oasmaa, A.; Solantausta, Y.; Arpiainen, V.; Kuoppala, E.; Sipilä, K. Fast pyrolysis bio-oils from wood and agricultural residues. Energy Fuels 2010, 2, 1380–1388. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of Fast Pyrolysis Oil Using Heterogeneous Noble-Metal Catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- App, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B 2012, 117–118, 105–117. [Google Scholar]
- Lu, Q.; Yang, X.-L.; Zhu, X.-F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Wildschut, J.; Iqbal, M.; Mahfud, F.H.; Cabrera, I.M.; Venderbosch, R.H.; Heeres, H.J. Insights in the hydrotreatment of fast pyrolysis oil using a ruthenium on carbon catalyst. Energy Environ. Sci. 2010, 3, 962–970. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwande, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic Hydroprocessing of Fast Pyrolysis Bio-oil from Pine Sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Elliott, D.C.; Neuenschwander, G.G. Liquid Fuels by Low-Severity Hydrotreating of Biocrude. In Developments in Thermochemical Biomass Conversion: Volume 1/Volume 2; Bridgwater, A.V., Boocock, D.G.B., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 611–621. [Google Scholar]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Fernández, C.; Gómez, X.; Martínez, O.; Sánchez, M.E. Thermogravimetric analysis of lignocellulosic and microalgae biomasses and their blends during combustion. J. Therm. Anal. 2013, 114, 295–305. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel 2019, 249, 441–448. [Google Scholar] [CrossRef]
- Plis, A.; Lasek, J.; Skawińska, A. Kinetic analysis of the combustion process of Nannochloropsis gaditana microalgae based on thermogravimetric studies. J. Anal. Appl. Pyrolysis 2017, 127, 109–119. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative Evaluation of Thermochemical Liquefaction and Pyrolysis for Bio-Oil Production from Microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, M.; Wang, K.; Zhang, L.; Xu, X. Catalytic Hydrodeoxygenation of Algae Bio-oil over Bimetallic Ni–Cu/ZrO2 Catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Gruia, A. Hydrotreating. In Handbook of Petroleum Processing; Jones, D.S.J.S., Pujado, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 321–354. [Google Scholar]
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-oil valorization: A review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Mauchausse, C.; Kural, E.; Trimm, D.L.; Cant, N.W. Optimization of tungsten-based catalysts for the hydrotreatment of coal-derived liquids. Fuel 1992, 71, 203–209. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Masel, R.I. Principles of Adsorption and Reaction on Solid Surfaces; Wiley: New York, NY, USA; Chichester, UK, 1996. [Google Scholar]
- Ptushinskiĭ, Y.G. Low-temperature adsorption of gases on metal surfaces (Review). Low Temp. Phys. 2004, 30, 1–26. [Google Scholar] [CrossRef]
- Ferrin, P.; Kandoi, S.; Nilekar, A.U.; Mavrikakis, M. Hydrogen adsorption, absorption and diffusion on and in transition metal surfaces: A DFT study. Surf. Sci. 2012, 606, 679–689. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hu, J.; Hart, T.R.; Neuenschwander, G.G. Palladium Catalyzed Hydrogenation of Bio-Oils and Organic Compounds. U.S. Patent 7,425,657, 16 September 2008. [Google Scholar]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Jensen, A.D. Screening of Catalysts for Hydrodeoxygenation of Phenol as a Model Compound for Bio-oil. ACS Catal. 2013, 3, 1774–1785. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Melián-Cabrera, I.V.; Heeres, H.J. Catalytic hydrotreatment of fast pyrolysis oil using bimetallic Ni–Cu catalysts on various supports. Appl. Catal. A 2012, 449, 121–130. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, Y.; Fan, G.; Yang, L.; Li, F. Structure-dependent selective hydrogenation of cinnamaldehyde over high-surface-area CeO2-ZrO2 composites supported Pt nanoparticles. Chem. Eng. J. 2017, 322, 234–245. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, P.; Wang, K.; Xu, J.; Jiang, J. Catalytic hydrogenation and one step hydrogenation-esterification to remove acetic acid for bio-oil upgrading: Model reaction study. Catal. Sci. Technol. 2016, 6, 7783–7792. [Google Scholar] [CrossRef]
- Bergem, H.; Xu, R.; Brown, R.C.; Huber, G.W. Low temperature aqueous phase hydrogenation of the light oxygenate fraction of bio-oil over supported ruthenium catalysts. Green Chem. 2017, 19, 3252–3262. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; van Haandel, L.; Ye, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Du, B.; Nasaruddin, R.R.; Chen, T.; Xie, J. Golden Carbon Nanotube Membrane for Continuous Flow Catalysis. Ind. Eng. Chem. Res. 2017, 56, 2999–3007. [Google Scholar] [CrossRef] [Green Version]
- Fiorio, J.L.; López, N.; Rossi, L.M. Gold–Ligand-Catalyzed Selective Hydrogenation of Alkynes into cis-Alkenes via H2 Heterolytic Activation by Frustrated Lewis Pairs. ACS Catal. 2017, 7, 2973–2980. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Characterization of Re–Pd/SiO2 Catalysts for Hydrogenation of Stearic Acid. ACS Catal. 2015, 5, 7034–7047. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Prakash, M.G.; Mahalakshmy, R.; Elangovan, T.; Viswanathan, B. Liquid phase hydrogenation of crotanaldehyde over nickel supported on titania. J. Mol. Catal. A Chem. 2013, 366, 92–98. [Google Scholar]
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd–Cu catalysts. Appl. Catal. A 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Yang, Y.; Chang, J.; Borgna, A. Hydrogenation of Furfural as Model Reaction of Bio-Oil Stabilization under Mild Conditions Using Multiwalled Carbon Nanotube (MWNT)-Supported Pt Catalysts. Ind. Eng. Chem. Res. 2014, 53, 11284–11291. [Google Scholar] [CrossRef]
- Ota, N.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Performance, Structure, and Mechanism of ReOx–Pd/CeO2 Catalyst for Simultaneous Removal of Vicinal OH Groups with H2. ACS Catal. 2016, 6, 3213–3226. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, R.; Tamura, M.; Tomishige, K. Combination of supported bimetallic rhodium–molybdenum catalyst and cerium oxide for hydrogenation of amide. Sci. Technol. Adv. Mater. 2015, 16, 014901. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Y.; Guo, J.; Zhao, L.; Hill, M.; Jiang, Z.; Zhao, Y. The Catalytic Hydrogenation of Maleic Anhydride on CeO2−δ-Supported Transition Metal Catalysts. Catalysts 2017, 7, 272. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Study of the hydrodeoxygenation of carbonyl, car☐ylic and guaiacyl groups over sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 catalysts: I. Catalytic reaction schemes. Appl. Catal. A 1994, 109, 77–96. [Google Scholar] [CrossRef]
- Bienholz, A.; Schwab, F.; Claus, P. Hydrogenolysis of glycerol over a highly active CuO/ZnO catalyst prepared by an oxalate gel method: Influence of solvent and reaction temperature on catalyst deactivation. Green Chem. 2010, 12, 290–295. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.; Jiang, T.; Han, B.; Zhou, Y. Hydrogenolysis of Glycerol to 1,2-Propanediol over Ru–Cu Bimetals Supported on Different Supports. CLEAN Soil Air Water 2012, 40, 318–324. [Google Scholar] [CrossRef]
- de Miguel Mercader, F.; Groeneveld, M.J.; Kersten, S.R.A.; Geantet, C.; Toussaint, G.; Way, N.W.J.; Schaverien, C.J.; Hogendoorn, K.J.A. Hydrodeoxygenation of pyrolysis oil fractions: Process understanding and quality assessment through co-processing in refinery units. Energy Environ. Sci. 2011, 4, 985–997. [Google Scholar] [CrossRef]
- Prasomsri, T.; Shetty, M.; Murugappan, K.; Román-Leshkov, Y. Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy Environ. Sci. 2014, 7, 2660–2669. [Google Scholar] [CrossRef]
- Bagnato, G.; Signoretto, M.; Pizzolitto, C.; Menegazzo, F.; Xi, X.; ten Brink, G.H.; Kooi, B.J.; Heeres, H.J.; Sanna, A. Hydrogenation of Biobased Aldehydes to Monoalcohols Using Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 11994–12004. [Google Scholar] [CrossRef]
- Sanna, A.; Vispute, T.P.; Huber, G.W. Hydrodeoxygenation of the aqueous fraction of bio-oil with Ru/C and Pt/C catalysts. Appl. Catal. B 2015, 165, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Oasmaa, A.; Kuoppala, E.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 2. Physicochemical Composition of Product Liquid. Energy Fuels 2003, 17, 433–443. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Gust, S.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 1. Effect of Extractives on Phase Separation of Pyrolysis Liquids. Energy Fuels 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part II: Comparative catalyst performance and reaction pathways. Fuel Process. Technol. 1995, 45, 185–202. [Google Scholar] [CrossRef]
- De la Puente, G.; Gil, A.; Pis, J.J.; Grange, P. Effects of Support Surface Chemistry in Hydrodeoxygenation Reactions over CoMo/Activated Carbon Sulfided Catalysts. Langmuir 1999, 15, 5800–5806. [Google Scholar] [CrossRef]
- Gutierrez, A.; Kaila, R.K.; Honkela, M.L.; Slioor, R.; Krause, A.O.I. Hydrodeoxygenation of guaiacol on noble metal catalysts. Catal. Today 2009, 147, 239–246. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Afanasiev, P.; Geantet, C. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity. Appl. Catal. B 2011, 101, 239–245. [Google Scholar] [CrossRef]
- Centeno, A.; Laurent, E.; Delmon, B. Influence of the Support of CoMo Sulfide Catalysts and of the Addition of Potassium and Platinum on the Catalytic Performances for the Hydrodeoxygenation of Carbonyl, Carboxyl, and Guaiacol-Type Molecules. J. Catal. 1995, 154, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Delmon, B.; Grange, P. Influence of the active phase loading in carbon supported molybdenum–cobalt catalysts for hydrodeoxygenation reactions. Microporous Mesoporous Mater. 2002, 56, 279–290. [Google Scholar] [CrossRef]
- Bindwal, A.B.; Bari, A.H.; Vaidya, P.D. Kinetics of low temperature aqueous-phase hydrogenation of model bio-oil compounds. Chem. Eng. J. 2012, 207–208, 725–733. [Google Scholar] [CrossRef]
- Bindwal, A.B.; Vaidya, P.D. Kinetics of Aqueous-Phase Hydrogenation of Levoglucosan over Ru/C Catalyst. Ind. Eng. Chem. Res. 2013, 52, 17781–17789. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Lumping Kinetic Model for Hydrotreating of Bio-oil from the Fast Pyrolysis of Biomass. Energy Sources Part A 2009, 31, 639–645. [Google Scholar] [CrossRef]
- Zhang, S.-P. Study of Hydrodeoxygenation of Bio-Oil from the Fast Pyrolysis of Biomass. Energy Sources 2003, 25, 57–65. [Google Scholar]
- Sheu, Y.-H.E.; Anthony, R.G.; Soltes, E.J. Kinetic studies of upgrading pine pyrolytic oil by hydrotreatment. Fuel Process. Technol. 1988, 19, 31–50. [Google Scholar] [CrossRef]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Longo, T.; Basile, A. Methanol steam reforming reaction in a Pd–Ag membrane reactor for CO-free hydrogen production. Int. J. Hydrogen Energy 2008, 33, 5583–5588. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. CO-free hydrogen production by steam reforming of acetic acid carried out in a Pd–Ag membrane reactor: The effect of co-current and counter-current mode. Int. J. Hydrogen Energy 2008, 33, 4091–4096. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, X.; Chen, R.; Liao, Q.; Feng, H.; Li, L. Catalytic membrane microreactor with Pd/γ-Al2O3 coated PDMS film modified by dopamine for hydrogenation of nitrobenzene. Chem. Eng. J. 2016, 301, 35–41. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Liao, S.; Yu, D. Mono- and bimetallic catalytic hollow- fiber reactors for the selective hydrogenation of butadiene in 1-butene. Appl. Catal. A Gen. 1998, 172, 23–29. [Google Scholar] [CrossRef]
- Stanford, J.P.; Soto, M.C.; Pfromm, P.H.; Rezac, M.E. Aqueous phase hydrogenation of levulinic acid using a porous catalytic membrane reactor. Catal. Today 2016, 268, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Bagnato, G.; Figoli, A.; Ursino, C.; Galiano, F.; Sanna, A. A novel Ru–polyethersulfone (PES) catalytic membrane for highly efficient and selective hydrogenation of furfural to furfuryl alcohol. J. Mater. Chem. A 2018, 6, 4955–4965. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P.; Giordano, C.; Sawa, H. Partial hydrogenation reactions over Pd-containing hybrid inorganic/polymeric catalytic membranes. Appl. Catal. A Gen. 2013, 459, 81–88. [Google Scholar] [CrossRef]
- Aran, H.C.; Klooster, H.; Jani, J.M.; Wessling, M.; Lefferts, L.; Lammertink, R.G.H. Influence of geometrical and operational parameters on the performance of porous catalytic membrane reactors. Chem. Eng. J. 2012, 207–208, 814–821. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Del Borghi, A.; Di Felice, R. Catalytic ceramic membrane in a three-phase reactor for the competitive hydrogenation–isomerisation of methylenecyclohexane. Sep. Purif. Technol. 2004, 34, 239–245. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Di Felice, R. Polymeric and ceramic membranes in three-phase catalytic membrane reactors for the hydrogenation of methylenecyclohexane. Desalination 2002, 144, 411–416. [Google Scholar] [CrossRef]
- Bengtson, G.; Fritsch, D. Catalytic membrane reactor for the selective hydrogenation of edible oil: Platinum versus palladium catalyst. Desalination 2006, 200, 666–667. [Google Scholar] [CrossRef] [Green Version]
- Liguori, F.; Barbaro, P.; Sawa, H. Continuous flow hydrogenation reactions by Pd catalysts onto hybrid ZrO2/PVA materials. Appl. Catal. A Gen. 2014, 488, 58–65. [Google Scholar] [CrossRef]









| Fraction/Chemical Groups | Compound Types | wt% (Wet Basis) [38] | wt% (Wet Basis) [39] | wt% [40] |
|---|---|---|---|---|
| Water solubles 75–85% | ||||
| Acids alcohols | Small acids, small alcohols | 5–10 | 6.5 | 8.5 |
| Ether-solubles | Catechols, syringols, guaiacols, aldehydes, ketones, furans, and pyrans | 5–15 | 15.4 | 20.3 |
| Ether-insolubles | Sugars | 30–40 | 34.4 | 45.3 |
| Water | Water | 20–30 | 23.9 | - |
| Water insoluble 15–25% | ||||
| Hexane-solubles | Extractives (High MW compounds with functional groups such as acids, alcohols) | 2–6 | 4.35 | 5.7 |
| DCM solubles | Stilbenes, Low MW lignin degraded compounds | 5–10 | 13.4 | 17.7 |
| DCM insolubles | High MW lignin degraded compounds | 2–10 | 1.95 | 2.6 |
| Feedstock for Bio-Oil | C | H | O | N | S | Ref. |
|---|---|---|---|---|---|---|
| Beechwood | 51.1 | 7.3 | 41.6 | [41] | ||
| Pine wood | 40.1 | 7.6 | 52.1 | 0.1 | [42] | |
| Rice husk | 39.92 | 8.15 | 51.29 | 0.61 | 0.03 | [43] |
| Beech wood | 58.6 | 6.2 | 35.2 | [44] | ||
| Pine sawdust | 38.8 | 7.7 | 53.4 | 0.09 | 0.02 | [45] |
| Eucalyptus | 44.8 | 7.2 | 48.1 | 0.2 | [46] | |
| White spruce | 49.6 | 6.4 | 43.1 | 0.2 | [47] | |
| Poplar | 49.5 | 6.05 | 44.4 | 0.07 | [47] | |
| Sawdust | 60.4 | 6.9 | 31.8 | 0.9 | [48] | |
| Microalgae | 54.8 | 7.6 | 28.7 | 8.5 | 0.4 | [49] |
| Scenedesmus | 44.6 | 6.1 | 40.8 | 4.8 | 3.6 | [50] |
| Nannochloropsis gaditana | 40.3 | 5.97 | 14.5 | 6.3 | 0.37 | [51] |
| Chlorella protothecoides | 62.1 | 8.7 | 11.2 | 9.7 | n/a | [52] |
| Spirulina | 67.5 | 9.8 | 11.3 | 10.7 | n/a | [53] |
| Nannochloropsis sp. | 80.2 | 6.2 | 5.8 | 6.2 | n/a | [54] |
| Catalyst | Reactant | Pressure (bar) | Temperature (°C) | Time (h) | Conversion (%) | Note | Ref. |
|---|---|---|---|---|---|---|---|
| 30% Ni/CNT | acetic acid | 40 | 150 | 4 | 5.8 | 2 wt% cat | [66] |
| 30% Cu/CNT | acetic acid | 40 | 150 | 4 | 3.5 | 2 wt% cat | [66] |
| Ru/C | acetic acid | 40 | 150 | 4 | 4.7 | 2 wt% cat | [66] |
| 20% Mo/CNT | acetic acid | 40 | 150 | 4 | <2 | 2 wt% cat | [66] |
| 10/10 wt% NiMo/CNT | acetic acid | 40 | 150 | 4 | 14.8 | 2 wt% cat | [66] |
| 3 wt% Ru/TiO2 | acetic acid | 62 | 120 | 33 * | 37.5 | * time on stream | [67] |
| 3 wt% Ru/TiO2 | Acetol | 62 | 70 | 14 * | 93.6 | * time on stream | [67] |
| 3 wt% Ru/TiO2 | Bio-oil | 62 | 120 | 21 | 27/38/79 ** | ** acetic acid/acetol/formic acid | [67] |
| 3 wt% Ru/C | Bio-oil | 52 | 120 | 6 | 33/99/97 ** | [67] | |
| Ru/Zr-MOFs | Furfural | 5 | 20 | 5 | 20–95 | TOF: 2–11 Selectivity to Furfuryl alcohol: 20–95 | [68] |
| AuNCs/CNTs membrane | 4-nitro-phenol | 53/100 | 5/10 µmol Au/17 cm2 | [69] | |||
| Au/SiO2 | 25 compounds | 80 | 6 | 5–24 | 40–99 | 1 mmol of alkyne, 0.01 mmol of Au, and 1 mmol of piperazine | [70] |
| Re–Pd/SiO2 | Stearic acid | 80 | 140 | 1 | 15 | Re/Pd = 1/8 | [71] |
| Re–Pd/SiO2 | Stearic acid | 80 | 140 | 4 | 13 | [71] | |
| Ni/rutile | Crotonaldehyde | 10 | 70 | 60 | [72] | ||
| Pd-Cu/MgO | Furfural | 6–8 | 80–130 | 0.5 | 100 | 98.7% selectivity of Furfuryl alcohol | [73] |
| Pt/MWNT | Furfural | 20 | 150 | 5 | 75–100 | Max Furfuryl alcohol selectivity: 79% | [74] |
| ReOx–Pd/CeO2 | 16 compounds | 80 | 140 | 4 | 1–60 | substrate 0.5 g, 1,4 dioxane 4 g, Wcat = 150 mg (2 wt% Re, 0.3 wt% Pd) | [75] |
| Rh–MoOx/SiO2+ CeO2 | cyclohexanecarboxamide | 80 | 140 | 4 | 89 | [76] |
| Hydrogenation of | Catalyst | Support | Pressure (bar) | Temperature (°C) | Ref. | |
|---|---|---|---|---|---|---|
| 3-hexyn-1-ol | Pd nanoparticles (4.6 nm) | zirconia/polyvinyl alcohol | Batch | 5–10 | 25 | [106] |
| Nitrite | Pd | γ-Al2O3 | Continuous | 1 | 25 | [107] |
| Methylenecyclohexane (and isomerization) | Pt, Pd, Ru in γ-Al2O3 | macroporous α-Al2O3 | Continuous | 1.5 liquid 2 gas | 15–70 | [108] |
| Methylenecyclohexane | Pd-PVDS PVP | macroporous α-Al2O3 | Continuous | 25–50 | [109] * | |
| Edible oil | Pd, Pt | porous polyamideimide (PAI) | Continuous | 4 | 100 | [110] |
| Nitrobenzene | Pd | zirconia/polyvinyl alcohol | Continuous | 1–2 | 25 | [111] |
| Nitrobenzene | Pd/γ-Al2O3 | PDMS | Continuous | 1–2 | 20 | [102] |
| Butadiene | PVP-Pd, PVP-Pd, EC-Pd, AR-Pd, AR-Pd, PVP-Pd, PVP-Pd-0.5 Co(OAc)2, PVP-Pd-0.5 Co(OAc)2 | CA, PSF, CA, CA, PSF, CA, CA, CA | Continuous | 10 | 40 | [103] ** |
| Furfural | Ru | PES | Continous | 7 | 70 | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnato, G.; Sanna, A.; Paone, E.; Catizzone, E. Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil. Catalysts 2021, 11, 157. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020157
Bagnato G, Sanna A, Paone E, Catizzone E. Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil. Catalysts. 2021; 11(2):157. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020157
Chicago/Turabian StyleBagnato, Giuseppe, Aimaro Sanna, Emilia Paone, and Enrico Catizzone. 2021. "Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil" Catalysts 11, no. 2: 157. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020157