Mesoporous Methyl-Functionalized Titanosilicate Produced by Aerosol Process for the Catalytic Epoxidation of Olefins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessing the Degree of Methyl-Functionalization and Hydrophobicity
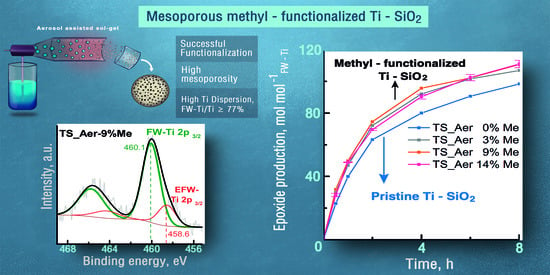
2.2. Quantification of Ti Dispersion
2.3. Catalytic Performance of Hydrophobic Mesoporous Ti-SiO2 on the Epoxidation of Cyclooctene
3. Materials and Methods
3.1. Preparation of Methyl-Functionalized Mesoporous Titanosilicate via Aerosol-Assisted Sol-Gel
3.2. Catalyst Characterization
3.3. Catalytic Epoxidation of Cyclooctene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the Alkyl−Alkoxide Precursor on the Structure and Catalytic Properties of Hybrid Sol−Gel Catalysts. Chem. Mater. 2005, 17, 6686–6694. [Google Scholar] [CrossRef]
- Swalus, C.; Farin, B.; Gillard, F.; Devillers, M.; Gaigneaux, E.M. Hybrid peroxotungstophosphate organized catalysts highly active and selective in alkene epoxidation. Catal. Commun. 2013, 37, 80–84. [Google Scholar] [CrossRef]
- Cordon, M.J.; Harris, J.W.; Vega-Vila, J.C.; Bates, J.S.; Kaur, S.; Gupta, M.; Witzke, M.E.; Wegener, E.C.; Miller, J.T.; Flaherty, D.W.; et al. Dominant Role of Entropy in Stabilizing Sugar Isomerization Transition States within Hydrophobic Zeolite Pores. J. Am. Chem. Soc. 2018, 140, 14244–14266. [Google Scholar] [CrossRef] [PubMed]
- Vivian, A.; Fusaro, L.; Debecker, D.P.; Aprile, C. Mesoporous Methyl-Functionalized Sn-Silicates Generated by the Aerosol Process for the Sustainable Production of Ethyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 14095–14103. [Google Scholar] [CrossRef]
- Chen, X.; Qian, P.; Zhang, T.; Xu, Z.; Fang, C.; Xu, X.; Chen, W.; Wu, P.; Shen, Y.; Li, S.; et al. Catalyst surfaces with tunable hydrophilicity and hydrophobicity: Metal-organic frameworks toward controllable catalytic selectivity. Chem. Commun. 2018, 54, 3936–3939. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Zhang, H.; Zhou, X.; An, B.; Lin, Z.; Dai, R.; Li, J.; Wang, C.; Lin, W. Surface Modification of Two-Dimensional Metal–Organic Layers Creates Biomimetic Catalytic Microenvironments for Selective Oxidation. Angew. Chem. Int. Ed. 2017, 56, 9704–9709. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Chen, S.; Hou, X.; Liu, B.; Lu, J.; Jiang, H.-L. Boosting selective oxidation of cyclohexane over a metal–organic framework by hydrophobicity engineering of pore walls. Chem. Commun. 2017, 53, 10026–10029. [Google Scholar] [CrossRef]
- Sun, Q.; He, H.; Gao, W.-Y.; Aguila, B.; Wojtas, L.; Dai, Z.; Li, J.; Chen, Y.-S.; Xiao, F.; Ma, S. Imparting amphiphobicity on single-crystalline porous materials. Nat. Commun. 2016, 7, 13300. [Google Scholar] [CrossRef] [Green Version]
- Khouw, C.; Dartt, C.; Labinger, J.; Davis, M. Studies on the Catalytic-Oxidation of Alkanes and Alkenes by Titanium Silicates. J. Catal. 1994, 149, 195–205. [Google Scholar] [CrossRef]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic Zeolites for Biofuel Upgrading Reactions at the Liquid–Liquid Interface in Water/Oil Emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef]
- Zapata, P.A.; Huang, Y.; Gonzalez-Borja, M.A.; Resasco, D.E. Silylated hydrophobic zeolites with enhanced tolerance to hot liquid water. J. Catal. 2013, 308, 82–97. [Google Scholar] [CrossRef]
- Gounder, R. Hydrophobic microporous and mesoporous oxides as Brønsted and Lewis acid catalysts for biomass conversion in liquid water. Catal. Sci. Technol. 2014, 4, 2877–2886. [Google Scholar] [CrossRef]
- Smeets, V.; Ben Mustapha, L.; Schnee, J.; Gaigneaux, E.M.; Debecker, D.P. Mesoporous SiO2-TiO2 epoxidation catalysts: Tuning surface polarity to improve performance in the presence of water. Mol. Catal. 2018, 452, 123–128. [Google Scholar] [CrossRef]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent No. 4,410,501, 18 October 1983. [Google Scholar]
- Notari, B. Synthesis and Catalytic Properties of Titanium Containing Zeolites. In Studies in Surface Science and Catalysis; Grobet, P.J., Mortier, W.J., Vansant, E.F., Schulz-Ekloff, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 37, pp. 413–425. [Google Scholar]
- Clerici, M.G. The activity of titanium silicalite-1 (TS-1): Some considerations on its origin. Kinet. Catal. 2015, 56, 450–455. [Google Scholar] [CrossRef]
- Signorile, M.; Crocellà, V.; Damin, A.; Rossi, B.; Lamberti, C.; Bonino, F.; Bordiga, S. Effect of Ti Speciation on Catalytic Performance of TS-1 in the Hydrogen Peroxide to Propylene Oxide Reaction. J. Phys. Chem. C 2018, 122, 9021–9034. [Google Scholar] [CrossRef]
- Bregante, D.T.; Thornburg, N.E.; Notestein, J.M.; Flaherty, D.W. Consequences of Confinement for Alkene Epoxidation with Hydrogen Peroxide on Highly Dispersed Group 4 and 5 Metal Oxide Catalysts. ACS Catal. 2018, 8, 2995–3010. [Google Scholar] [CrossRef]
- Corma, A.; Esteve, P.; Martínez, A. Solvent Effects during the Oxidation of Olefins and Alcohols with Hydrogen Peroxide on Ti-Beta Catalyst: The Influence of the Hydrophilicity-Hydrophobicity of the Zeolite. J. Catal. 1996, 161, 11–19. [Google Scholar] [CrossRef]
- Cordeiro, P.J.; Tilley, T.D. Enhancement of the Catalytic Activity of Titanium-Based Terminal Olefin Epoxidation Catalysts via Surface Modification with Functionalized Protic Molecules. ACS Catal. 2011, 1, 455–467. [Google Scholar] [CrossRef]
- Wu, P.; Tatsumi, T.; Komatsu, T.; Yashima, T. Postsynthesis, Characterization, and Catalytic Properties in Alkene Epoxidation of Hydrothermally Stable Mesoporous Ti-SBA-15. Chem. Mater. 2002, 14, 1657–1664. [Google Scholar] [CrossRef]
- Chiker, F.; Launay, F.; Nogier, J.P.; Bonardet, J.L. Green and selective epoxidation of alkenes catalysed by new TiO2–SiO2SBA mesoporous solids. Green Chem. 2003, 5, 318–322. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Navarro, M.A.; Pariente, J.P. Synthesis, Characterization, and Catalytic Activity of Ti-MCM-41 Structures. J. Catal. 1995, 156, 65–74. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.; Gaona, J.A.; Jorda, J.L.; Navarro, M.T.; Rey, F.; Pérez-Pariente, J.; Tsuji, J.; McCulloch, B.; Nemeth, L.T. Strategies to improve the epoxidation activity and selectivity of Ti-MCM-41. Chem. Commun. 1998, 2211–2212. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Vispe, E.; Brown, D.R.; Naderi, M. Is MCM-41 really advantageous over amorphous silica? The case of grafted titanium epoxidation catalysts. Chem. Commun. 2001, 10, 1510–1511. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Z.; Xu, L.; Li, M.; Xin, Q.; Liu, Z.; Li, C. Ti−MCM-41 Synthesized from Colloidal Silica and Titanium Trichloride: Synthesis, Characterization, and Catalysis. Chem. Mater. 2001, 13, 994–998. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Vispe, E. Silica-Supported Titanium Derivatives as Catalysts for the Epoxidation of Alkenes with Hydrogen Peroxide: A New Way to Tuneable Catalytic Activity through Ligand Exchange. J. Catal. 2000, 189, 40–51. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Vispe, E. Effect of the Reaction Conditions on the Epoxidation of Alkenes with Hydrogen Peroxide Catalyzed by Silica-Supported Titanium Derivatives. J. Catal. 2001, 204, 146–156. [Google Scholar] [CrossRef]
- Smeets, V.; Boissière, C.; Sanchez, C.; Gaigneaux, E.M.; Peeters, E.; Sels, B.F.; Dusselier, M.; Debecker, D.P. Aerosol Route to TiO2–SiO2Catalysts with Tailored Pore Architecture and High Epoxidation Activity. Chem. Mater. 2019, 31, 1610–1619. [Google Scholar] [CrossRef]
- Beck, C.; Mallat, T.; Baiker, A. On the Limited Selectivity of Silica-Based Epoxidation Catalysts. Catal. Lett. 2001, 75, 131–136. [Google Scholar] [CrossRef]
- Beck, C.; Mallat, T.; Buergi, T.; Baiker, A. Nature of Active Sites in Sol-Gel TiO2–SiO2 Epoxidation Catalysts. J. Catal. 2001, 204, 428–439. [Google Scholar] [CrossRef]
- Hutter, R.; Mallat, T.; Baiker, A. Titania Silica Mixed Oxides. J. Catal. 1995, 153, 177–189. [Google Scholar] [CrossRef]
- Hutter, R.; Mallat, T.; Dutoit, D.; Baiker, A. Titania-silica aerogels with superior catalytic performance in olefin epoxidation compared to large pore Ti-molecular sieves. Top. Catal. 1996, 3, 421–436. [Google Scholar] [CrossRef]
- Dutoit, D.; Schneider, M.; Hutter, R.; Baiker, A. Titania–Silica Mixed Oxides. J. Catal. 1996, 161, 651–658. [Google Scholar] [CrossRef]
- Bordiga, S.; Coluccia, S.; Lamberti, C.; Marchese, L.; Zecchina, A.; Boscherini, F.; Buffa, F.; Genoni, F.; Leofanti, G. XAFS Study of Ti-Silicalite: Structure of Framework Ti(IV) in the Presence and Absence of Reactive Molecules (H2O, NH3) and Comparison with Ultraviolet-Visible and IR Results. J. Phys. Chem. 1994, 98, 4125–4132. [Google Scholar] [CrossRef]
- Oyama, S.T. Chapter 1—Rates, Kinetics, and Mechanisms of Epoxidation: Homogeneous, Heterogeneous, and Biological Routes. In Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–99. [Google Scholar] [CrossRef]
- Clerici, M.G.; Kholdeeva, O.A. Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gao, X.; Wachs, I.E. Titania–silica as catalysts: Molecular structural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Thomas, J.M.; Sankar, G.; Klunduk, M.C.; Attfield, M.P.; Maschmeyer, T.; Johnson, B.F.G.; Bell, R.G. The Identity in Atomic Structure and Performance of Active Sites in Heterogeneous and Homogeneous, Titanium−Silica Epoxidation Catalysts. J. Phys. Chem. B 1999, 103, 8809–8813. [Google Scholar] [CrossRef]
- Bregante, D.T.; Flaherty, D.W. Periodic Trends in Olefin Epoxidation over Group IV and V Framework-Substituted Zeolite Catalysts: A Kinetic and Spectroscopic Study. J. Am. Chem. Soc. 2017, 139, 6888–6898. [Google Scholar] [CrossRef]
- Liang, X.; Mi, Z.; Wu, Y.; Wang, L.; Xing, E. Kinetics of epoxidation of propylene over TS-1 in isopropanol. React. Kinet. Catal. Lett. 2003, 80, 207–215. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Tilley, T.D. Kinetics and Mechanism of Olefin Epoxidation with Aqueous H2O2and a Highly Selective Surface-Modified TaSBA15 Heterogeneous Catalyst. J. Am. Chem. Soc. 2008, 130, 11088–11096. [Google Scholar] [CrossRef]
- Thangaraj, A.; Sivasanker, S. An improved method for TS-1 synthesis: 29Si NMR studies. J. Chem. Soc. Chem. Commun. 1992, 10, 123–124. [Google Scholar] [CrossRef]
- Notestein, J.M.; Iglesia, E.; Katz, A. Grafted Metallocalixarenes as Single-Site Surface Organometallic Catalysts. J. Am. Chem. Soc. 2004, 126, 16478–16486. [Google Scholar] [CrossRef]
- Fan, W.; Duan, R.-G.; Yokoi, T.; Wu, P.; Kubota, Y.; Tatsumi, T. Synthesis, Crystallization Mechanism, and Catalytic Properties of Titanium-Rich TS-1 Free of Extraframework Titanium Species. J. Am. Chem. Soc. 2008, 130, 10150–10164. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wu, P.; Tatsumi, T. Unique solvent effect of microporous crystalline titanosilicates in the oxidation of 1-hexene and cyclohexene. J. Catal. 2008, 256, 62–73. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Vispe, E. Optimization of cyclohexene epoxidation with dilute hydrogen peroxide and silica-supported titanium catalysts. Appl. Catal. A Gen. 2003, 245, 363–376. [Google Scholar] [CrossRef]
- Langhendries, G.; De Vos, D.E.; Baron, G.V.; Jacobs, P.A. Quantitative Sorption Experiments on Ti-Zeolites and Relation with α-Olefin Oxidation by H2O2. J. Catal. 1999, 187, 453–463. [Google Scholar] [CrossRef]
- Blasco, T.; Camblor, M.A.; Corma, A.; Esteve, P.; Guil, J.M.; Martínez, A.; Perdigón-Melón, A.J.A.; Valencia, S. Direct Synthesis and Characterization of Hydrophobic Aluminum-Free Ti−Beta Zeolite. J. Phys. Chem. B 1998, 102, 75–88. [Google Scholar] [CrossRef]
- Blasco, T.; Camblor, M.A.; Corma, A.; Esteve, P.; Martínez, A.; Prieto, C.; Valencia, S. Unseeded synthesis of Al-free Ti-β zeolite in fluoride medium: A hydrophobic selective oxidation catalyst. Chem. Commun. 1996, 10, 2367–2368. [Google Scholar] [CrossRef]
- Tatsumi, T.; Koyano, K.A.; Igarashi, N. Remarkable activity enhancement by trimethylsilylation in oxidation of alkenes and alkanes with H2O2 catalyzed by titanium-containing mesoporous molecular sieves. Chem. Commun. 1998, 325–326. [Google Scholar] [CrossRef]
- Brutchey, R.L.; Ruddy, D.A.; Andersen, L.K.; Tilley, T.D. Influence of Surface Modification of Ti−SBA15 Catalysts on the Epoxidation Mechanism for Cyclohexene with Aqueous Hydrogen Peroxide. Langmuir 2005, 21, 9576–9583. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Xiao, L.; Fan, J.; Zheng, X. Fabrication of Super-Hydrophobic Titanosilicate Sub-micro Sphere with Enhanced Epoxidation Catalytic Activity. Catal. Lett. 2019, 149, 1396–1402. [Google Scholar] [CrossRef]
- Guo, Y.; Hwang, S.-J.; Katz, A. Hydrothermally robust Ti/SiO2 epoxidation catalysts via surface modification with oligomeric PMHS. Mol. Catal. 2019, 477, 110509. [Google Scholar] [CrossRef]
- D’Amore, M.B.; Schwarz, S. Trimethylsilylation of ordered and disordered titanosilicates: Improvements in epoxidation with aqueous H2O2 from micro- to meso-pores and beyond†. Chem. Commun. 1999, 121–122. [Google Scholar] [CrossRef]
- Figueras, F.; Kochkar, H. Effects of hydrophobicity on the epoxidation of cyclohexene by tert-butyl hydroperoxide on TiO2–SiO2 mixed oxides. Catal. Lett. 1999, 59, 79–81. [Google Scholar] [CrossRef]
- Kochkar, H.; Figueras, F. Synthesis of Hydrophobic TiO2–SiO2Mixed Oxides for the Epoxidation of Cyclohexene. J. Catal. 1997, 171, 420–430. [Google Scholar] [CrossRef]
- Lin, K.; Pescarmona, P.P.; Houthoofd, K.; Liang, D.; Van Tendeloo, G.; Jacobs, P.A. Direct room-temperature synthesis of methyl-functionalized Ti-MCM-41 nanoparticles and their catalytic performance in epoxidation. J. Catal. 2009, 263, 75–82. [Google Scholar] [CrossRef]
- Klein, S.; Maier, W.F. Microporous Mixed Oxides—Catalysts with Tunable Surface Polarity. Angew. Chem. Int. Ed. 1996, 35, 2230–2233. [Google Scholar] [CrossRef]
- Müller, C.; Deck, R.; Mallat, T.; Baiker, A. Hydrophobic titania–silica aerogels: Epoxidation of cyclic compounds. Top. Catal. 2000, 11, 369–378. [Google Scholar] [CrossRef]
- Müller, C.; Maciejewski, M.; Mallat, T.; Baiker, A. Organically Modified Titania–Silica Aerogels for the Epoxidation of Olefins and Allylic Alcohols. J. Catal. 1999, 184, 280–293. [Google Scholar] [CrossRef]
- Perugachi, L.E.M.; Vivian, A.; Eloy, P.; Debecker, D.P.; Aprile, C.; Gaigneaux, E.M. Hydrophobic titania-silica mixed oxides for the catalytic epoxidation of cyclooctene. Catal. Today 2019. [Google Scholar] [CrossRef]
- Debecker, D.P.; Le Bras, S.; Boissière, C.; Chaumonnot, A.; Sanchez, C. Aerosol processing: A wind of innovation in the field of advanced heterogeneous catalysts. Chem. Soc. Rev. 2018, 47, 4112–4155. [Google Scholar] [CrossRef]
- Godard, N.; Vivian, A.; Fusaro, L.; Cannavicci, L.; Aprile, C.; Debecker, D.P. High-Yield Synthesis of Ethyl Lactate with Mesoporous Tin Silicate Catalysts Prepared by an Aerosol-Assisted Sol-Gel Process. ChemCatChem 2017, 9, 2211–2218. [Google Scholar] [CrossRef]
- Kim, A.; Sanchez, C.; Haye, B.; Boissière, C.; Sassoye, C.; Debecker, D.P. Mesoporous TiO2 Support Materials for Ru-Based CO2 Methanation Catalysts. ACS Appl. Nano Mater. 2019, 2, 3220–3230. [Google Scholar] [CrossRef]
- Wang, C.Y.; Bai, H. Aerosol processing of mesoporous silica supported bimetallic catalysts for low temperature acetone oxidation. Catal. Today 2011, 174, 70–78. [Google Scholar] [CrossRef]
- Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Direct Aerosol Synthesis of Large-Pore Amorphous Mesostructured Aluminosilicates with Superior Acid-Catalytic Properties. Angew. Chem. Int. Ed. 2009, 48, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Maksasithorn, S.; Praserthdam, P.; Suriye, K.; Debecker, D.P. Preparation of super-microporous WO3–SiO2 olefin metathesis catalysts by the aerosol-assisted sol-gel process. Microporous Mesoporous Mater. 2015, 213, 125–133. [Google Scholar] [CrossRef]
- Oveisi, H.; Suzuki, N.; Beitollahi, A.; Yamauchi, Y. Aerosol-assisted fabrication of mesoporous titania spheres with crystallized anatase structures and investigation of their photocatalitic properties. J. Sol. Gel Sci. Technol. 2010, 56, 212–218. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Y.; Wang, J.; Dong, X.; Tian, Q.; Han, Y. Recent progress in the direct synthesis of hierarchical zeolites: Synthetic strategies and characterization methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bregante, D.T.; Johnson, A.M.; Patel, A.Y.; Ayla, E.Z.; Cordon, M.J.; Bukowski, B.C.; Greeley, J.P.; Gounder, R.; Flaherty, D.W. Cooperative Effects between Hydrophilic Pores and Solvents: Catalytic Consequences of Hydrogen Bonding on Alkene Epoxidation in Zeolites. J. Am. Chem. Soc. 2019, 141, 7302–7319. [Google Scholar] [CrossRef]
- Olson, D.; Haag, W.; Borghard, W. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Microporous Mesoporous Mater. 2000, 35-36, 435–446. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Zecchina, A.; Spoto, G.; Bordiga, S.; Ferrero, A.; Petrini, G.; Leofanti, G.; Padovan, M. Framework and Extraframework Ti in Titanium-Silicalite: Investigation by Means of Physical Methods. In Studies in Surface Science and Catalysis; Elsevier BV: Amsterdam, The Netherlands, 1991; Volume 69, pp. 251–258. [Google Scholar]
- Klein, S.; Weckhuysen, B.; Martens, J.; Maier, W.; Jacobs, P.A. Homogeneity of Titania-Silica Mixed Oxides: On UV-DRS Studies as a Function of Titania Content. J. Catal. 1996, 163, 489–491. [Google Scholar] [CrossRef]
- Ikeue, K.; Ikeda, S.; Watanabe, A.; Ohtani, B. Elucidation of the local structure of active titanium(iv) sites on silica-based phase-boundary catalysts for alkene epoxidation with aqueous hydrogen peroxide. Phys. Chem. Chem. Phys. 2004, 6, 2523–2528. [Google Scholar] [CrossRef] [Green Version]
- On, D.T.; Bonneviot, L.; Le Noc, L. Electron transfer bands of titanium sites in dehydrated silicalites and in TiO2–SiO2gel. Chem. Commun. 1996, 10, 299–300. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Poleunis, C.; Debecker, D.P.; Giebeler, L.; Oswald, S.; Makshina, E.; Sels, B.F. Titania-Silica Catalysts for Lactide Production from Renewable Alkyl Lactates: Structure–Activity Relations. ACS Catal. 2018, 8, 8130–8139. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi (b) 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Wagner, C.; Riggs, W.; Davis, L.; Moulder, J.; Muilenberg, G. Handbook of X-Ray Photoelectron Spectroscopy; Muilenberg, G., Ed.; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Stakheev, A.Y.; Shpiro, E.S.; Apijok, J. XPS and XAES study of titania-silica mixed oxide system. J. Phys. Chem. 1993, 97, 5668–5672. [Google Scholar] [CrossRef]
- Tyablikov, I.A.; Rodionova, L.I.; Sobolev, P.D.; Ivanova, I.I. Formation of active sites in titanium-containing zeolites with MFI structure in propylene epoxidation with hydrogen peroxide. Pet. Chem. 2016, 56, 267–274. [Google Scholar] [CrossRef]
- Yamashita, H.; Kawasaki, S.; Ichihashi, Y.; Harada, M.; Takeuchi, A.M.; Anpo, M.; Stewart, G.; Fox, M.A.; Louis, C.; Che, M. Characterization of Titanium–Silicon Binary Oxide Catalysts Prepared by the Sol-Gel Method and Their Photocatalytic Reactivity for the Liquid-Phase Oxidation of 1-Octanol. J. Phys. Chem. B 1998, 102, 5870–5875. [Google Scholar] [CrossRef]
- Langerame, F.; Salvi, A.M.; Silletti, M.; Moretti, G. XPS characterization of a synthetic Ti-containing MFI zeolite framework: The titanosilicalites, TS-1. Surf. Interface Anal. 2008, 40, 695–699. [Google Scholar] [CrossRef]
- Vetter, S.; Schulz-Ekloff, G.; Kulawik, K.; Jaeger, N.I. On the para/ortho product ratio of phenol and anisole hydroxylation over titanium silicalite-1. Chem. Eng. Technol. 1994, 17, 348–353. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ayame, A. Investigation of oxidation states of titanium in titanium silicalite-1 by X-ray photoelectron spectroscopy. Catal. Today 2001, 71, 177–187. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]











| Sample | SSAcorrected a m2 g−1 | Pore volume (77 K) b cm3 g−1 | Micropore volume (77 K) c cm3 g−1 |
|---|---|---|---|
| TS_Aer-0%Me | 360 | 1.00 | 0.21 |
| TS_Aer-3%Me | 364 | 1.00 | 0.20 |
| TS_Aer-9%Me | 402 | 0.98 | 0.18 |
| TS_Aer-14%Me | 462 | 0.99 | 0.16 |
| Sample | Phys H2O/SSAcorrected a molH2O m−2 | Fraction of Si as Q3 and Q2 species b mole % | H2O Uptake at p/po = 0.1 c, molH2O m−2 | H2O Uptake at p/po = 0.2 c, molH2O m−2 |
|---|---|---|---|---|
| TS_Aer-0%Me | 22.3 | 32.1 | 5.1 | 11.6 |
| TS_Aer-3%Me | 13.2 | 31.5 | n.m. d | n.m. d |
| TS_Aer-9%Me | 9.3 | 30.7 | 5.1 | 9.1 |
| TS_Aer-14%Me | 13.1 | 24.2 | 3.4 | 6.1 |
| Sample | Bulk Ti/(Si+Ti) at. Ratio | Surf. Ti/(Si+Ti) at. Ratio | Surf. FW-Ti/(Si+Ti) at. Ratio | Surf. FW-Ti/Ti at. Ratio | Surf. C/(Si+Ti) at. Ratio | Surf. C–H/Si at. Ratio |
|---|---|---|---|---|---|---|
| TS_Aer-0%Me | 0.020 | 0.015 | 0.013 | 0.85 | 0.227 | 0.133 |
| TS_Aer-3%Me | 0.020 | 0.016 | 0.013 | 0.83 | 0.282 | 0.191 |
| TS_Aer-9%Me | 0.020 | 0.017 | 0.014 | 0.79 | 0.306 | 0.205 |
| TS_Aer-14%Me | 0.020 | 0.019 | 0.014 | 0.77 | 0.383 | 0.273 |
| Sample | Ti atoms·nm−2 * | FW-Ti atoms·nm−2 * |
|---|---|---|
| TS_Aer-0%Me | 0.37 | 0.32 |
| TS_Aer-3%Me | 0.40 | 0.32 |
| TS_Aer-9%Me | 0.37 | 0.31 |
| TS_Aer-14%Me | 0.37 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manangon-Perugachi, L.E.; Smeets, V.; Vivian, A.; Kainthla, I.; Eloy, P.; Aprile, C.; Debecker, D.P.; Gaigneaux, E.M. Mesoporous Methyl-Functionalized Titanosilicate Produced by Aerosol Process for the Catalytic Epoxidation of Olefins. Catalysts 2021, 11, 196. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020196
Manangon-Perugachi LE, Smeets V, Vivian A, Kainthla I, Eloy P, Aprile C, Debecker DP, Gaigneaux EM. Mesoporous Methyl-Functionalized Titanosilicate Produced by Aerosol Process for the Catalytic Epoxidation of Olefins. Catalysts. 2021; 11(2):196. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020196
Chicago/Turabian StyleManangon-Perugachi, Lucia E., Valentin Smeets, Alvise Vivian, Itika Kainthla, Pierre Eloy, Carmela Aprile, Damien P. Debecker, and Eric M. Gaigneaux. 2021. "Mesoporous Methyl-Functionalized Titanosilicate Produced by Aerosol Process for the Catalytic Epoxidation of Olefins" Catalysts 11, no. 2: 196. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020196