Biocatalytic Transformation of 5-Hydroxymethylfurfural into 2,5-di(hydroxymethyl)furan by a Newly Isolated Fusarium striatum Strain
Abstract
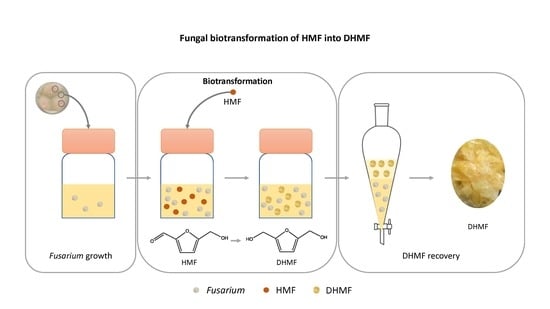
:1. Introduction
2. Results
2.1. Fusarium Screening
2.2. Effect of the Concentration of HMF
2.3. Effect of the Inoculum Size, Glucose Concentration and pH
2.4. Inoculation with Spores
2.5. Substrate Feeding Approach
2.6. Scale-up of the Reaction
2.7. Bioreactor
2.8. Recovery of the DHMF from the Reaction Broth
3. Materials and Methods
3.1. Materials
3.2. Cultivation of Fusarium Cells
3.3. Screening
3.4. Biotransformation Experiments
3.5. Effect of Inoculum Size, Glucose Concentration and pH
3.6. Sporulation of F. Striatum
3.7. Scale-up in the Shake Flasks
3.8. Scale-up in the Bioreactor
3.9. Recovery of DHMF from the Reaction Media
3.10. GC-FID Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liguori, R.; Amore, A.; Faraco, V. Waste valorization by biotechnological conversion into added value products. Appl. Microbiol. Biotechnol. 2013, 97, 6129–6147. [Google Scholar] [CrossRef]
- Ali, S.S.; Nugent, B.; Mullins, E.; Doohan, F.M. Fungal-mediated consolidated bioprocessing: The potential of Fusarium oxysporum for the lignocellulosic ethanol industry. AMB Express 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, J.; Su, D. 5-Hydroxymethylfurfural: A key intermediate for efficient biomass conversion. J. Energy Chem. 2015, 24, 548–551. [Google Scholar] [CrossRef]
- Hu, L.; He, A.; Liu, X.; Xia, J.; Xu, J.; Zhou, S.; Xu, J. Biocatalytic Transformation of 5-Hydroxymethylfurfural into High-Value Derivatives: Recent Advances and Future Aspects. ACS Sustain. Chem. Eng. 2018, 6, 15915–15935. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Bertilsson, M.; Gorwa-Grauslund, M.-F.; Gorsich, S.; Lidén, G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Ran, H.; Zhang, J.; Gao, Q.; Lin, Z.; Bao, J. Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnol. Biofuels 2014, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Feldman, D.; Kowbel, D.J.; Glass, N.L.; Yarden, O.; Hadar, Y. Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol. Biofuels 2015, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gao, Q.; Bao, J. Transcriptional analysis of Amorphotheca resinae ZN1 on biological degradation of furfural and 5-hydroxymethylfurfural derived from lignocellulose pretreatment. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Tang, X.; Peng, L.; Wei, J.; Lin, L. Methods in the Synthesis and Conversion of 2,5-Bis-(hydroxymethyl)furan from Bio-derived 5-Hydroxymethylfurfural and its Great Potential in Polymerization. BioResources 2018, 13, 7137–7154. [Google Scholar] [CrossRef]
- Maniar, D.; Jiang, Y.; Woortman, A.J.J.; Van Dijken, J.; Loos, K. Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef] [Green Version]
- De María, P.D.; Guajardo, N. Biocatalytic Valorization of Furans: Opportunities for Inherently Unstable Substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-C.; Jiang, C.-X.; Chong, G.-G.; Di, J.-H.; Ma, C.-L. Biological synthesis of 2,5-bis(hydroxymethyl)furan from biomass-derived 5-hydroxymethylfurfural by E. coli CCZU-K14 whole cells. Bioresour. Technol. 2018, 247, 1215–1220. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhang, X.-Y.; Li, N.; Xu, P.; Lou, W.-Y.; Zong, M.-H. Biocatalytic Reduction of HMF to 2,5-Bis(hydroxymethyl)furan by HMF-Tolerant Whole Cells. ChemSusChem 2017, 10, 372–378. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Cheng, A.-D.; Xing, X.-P.; Zong, M.-H.; Bai, Y.-P.; Li, N. Improved synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfural using acclimatized whole cells entrapped in calcium alginate. Bioresour. Technol. 2018, 262, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-H.; Zong, M.-H.; Li, N. Catalytic synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfual by recombinant Saccharomyces cerevisiae. Enzym. Microb. Technol. 2020, 134, 109491. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced Biotransformation of Furfural and Hydroxymethylfurfural by Newly Developed Ethanologenic Yeast Strains. Appl. Biochem. Biotechnol. 2005, 121, 451–460. [Google Scholar] [CrossRef]
- Ra, C.H.; Jeong, G.-T.; Shin, M.K.; Kim, S.-K. Biotransformation of 5-hydroxymethylfurfural (HMF) by Scheffersomyces stipitis during ethanol fermentation of hydrolysate of the seaweed Gelidium amansii. Bioresour. Technol. 2013, 140, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-K.; Qiao, X.-G.; Miao, C.-P.; Liu, K.; Chen, Y.-W.; Xu, L.-H.; Zhao, L.-X. Diversity, distribution and biotechnological potential of endophytic fungi. Ann. Microbiol. 2015, 66, 529–542. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, M.G.; Paulino, B.N.; Mano, M.C.R.; Neri-Numa, I.A.; Molina, G.; Pastore, G.M. Fusarium species—a promising tool box for industrial biotechnology. Appl. Microbiol. Biotechnol. 2017, 101, 3493–3511. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-J.; Yang, H.-R.; Wu, X.-L.; Peng, F.; Huang, Z.; Pu, L.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int. J. Biol. Macromol. 2019, 137, 568–575. [Google Scholar] [CrossRef]
- Moreira, M.T.; Feijoo, G.; Lema, J.M. Fungal Bioreactors: Applications to White-Rot Fungi. Rev. Environ. Sci. Bio Technol. 2003, 2, 247–259. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Herpoël-Gimbert, I.; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.Y.; Qi, Y.L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology 2012, 3, 195–200. [Google Scholar] [CrossRef]
- Stiborova, H.; Branska, B.; Vesela, T.; Lovecka, P.; Stranska, M.; Hajslova, J.; Jiru, M.; Patakova, P.; Demnerova, K. Transformation of raw feather waste into digestible peptides and amino acids. J. Chem. Technol. Biotechnol. 2016, 91, 1629–1637. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Fu, W.; Jensen, J.S.; Woodley, J.M. Process considerations for the scale-up and implementation of biocatalysis. Food Bioprod. Process. 2010, 88, 3–11. [Google Scholar] [CrossRef]






| Run | Coded Levels | Real Values | Responses | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Inoculum Size (Discs) | pH | [Glucose] (g × L−1) | mmol DHMF (24 h) | mmol DHMF (72 h) | |
| 1 | −1 | −1 | −1 | 3 | 5 | 20 | 0.81 ± 0.08 | 1.41 ± 0.13 |
| 2 | 1 | −1 | −1 | 6 | 5 | 20 | 0.96 ± 0.07 | 1.31 ± 0.21 |
| 3 | −1 | 1 | −1 | 3 | 7 | 20 | 0.85 ± 0.04 | 2.12 ± 0.31 |
| 4 | 1 | 1 | −1 | 6 | 7 | 20 | 1.02 ± 0.03 | 2.14 1 |
| 5 | −1 | −1 | 1 | 3 | 5 | 40 | 0.78 ± 0.07 | 1.18 ± 0.26 |
| 6 | 1 | −1 | 1 | 6 | 5 | 40 | 0.95 ± 0.02 | 1.31 ± 0.00 |
| 7 | −1 | 1 | 1 | 3 | 7 | 40 | 0.80 ± 0.09 | 1.58 ± 0.32 |
| 8 | 1 | 1 | 1 | 6 | 7 | 40 | 0.95 ± 0.06 | 1.90 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, A.; Sala, N.; Torres, M.; Canela-Garayoa, R. Biocatalytic Transformation of 5-Hydroxymethylfurfural into 2,5-di(hydroxymethyl)furan by a Newly Isolated Fusarium striatum Strain. Catalysts 2021, 11, 216. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020216
Millán A, Sala N, Torres M, Canela-Garayoa R. Biocatalytic Transformation of 5-Hydroxymethylfurfural into 2,5-di(hydroxymethyl)furan by a Newly Isolated Fusarium striatum Strain. Catalysts. 2021; 11(2):216. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020216
Chicago/Turabian StyleMillán, Alberto, Núria Sala, Mercè Torres, and Ramon Canela-Garayoa. 2021. "Biocatalytic Transformation of 5-Hydroxymethylfurfural into 2,5-di(hydroxymethyl)furan by a Newly Isolated Fusarium striatum Strain" Catalysts 11, no. 2: 216. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020216