Tailoring Properties of Metal-Free Catalysts for the Highly Efficient Desulfurization of Sour Gases under Harsh Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization and Properties of N-C2/SiC and N-C4/SiC
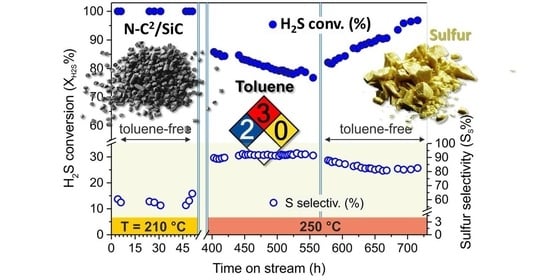
2.2. Desulfurization Performance of N-C2/SiC and N-C4/SiC in the Presence of a Relatively High Vol.% of Toluene as Acid Gas Contaminant
3. Experimental Section
3.1. Materials and Methods
3.2. General Procedure for the Preparation of N-C2/SiC and N-C4/SiC Catalysts
3.3. Selective H2S Desulfurization of Sour Gases to Elemental Sulfur
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comparison Against Other Fossil Fuels. Available online: https://www.swarthmore.edu/environmental-studies-capstone/comparison-against-other-fossil-fuels (accessed on 7 December 2020).
- Faramawy, S.; Zaki, T.; Sakr, A.A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Wieckowska, J. Catalytic and adsorptive desulphurization of gases. Catal. Today 1995, 24, 405–465. [Google Scholar] [CrossRef]
- Eow, J.S. Recovery of Sulfur from Sour Acid Gas: A Review of the Technology. Environ. Prog. 2002, 21, 143–162. [Google Scholar] [CrossRef]
- Piéplu, A.; Saur, O.; Lavalley, J.C.; Legendre, O.; Nédez, C. Claus Catalysis and H2S Selective Oxidation. Catal. Rev. Sci. Eng. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-Selective Catalytic Oxidation: Catalysts and Processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Ballaguet, J.-P.R.; Vaidya, M.M.; Duval, S.A.; Harale, A.; Khawajah, A.H.; Tammana, V.V.R. Sulfur Recovery Process for Treating Low to Medium Mole Percent Hydrogen Sulfide Gas Feeds with BTEX in a Claus Unit. U.S. Patent 9981848-B2, 29 May 2018. [Google Scholar]
- Bahadori, A. Natural Gas Processing Technology and Engineering Design, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2014. [Google Scholar]
- Jahangiri, M.; Shahtaheri, S.J.; Adl, J.; Rashidi, A.; Kakooei, H.; Forushani, A.R.; Ganjali, M.R.; Ghorbanali, A. The adsorption of benzene, toluene and xylenes (BTX) on the carbon nanostructures: The study of different parameters. Fresenius Environ. Bull. 2011, 20, 1036–1045. [Google Scholar]
- Sulphur Prices, Markets & Analysis. Available online: http://Www.Icis.Com/Fertilizers/Sulphur/ (accessed on 9 December 2020).
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Ba, H.; Luo, J.; Liu, Y.; Duong-Viet, C.; Tuci, G.; Giambastiani, G.; Nhut, J.-M.; Nguyen-Dinh, L.; Ersen, O.; Su, D.S.; et al. Macroscopically shaped monolith of nanodiamonds @nitrogen-enriched mesoporous carbon decorated SiC as a superiormetal-free catalyst for the styrene production. Appl. Catal. B Environ. 2017, 200, 343–350. [Google Scholar] [CrossRef]
- Diao, J.; Feng, Z.; Huang, R.; Liu, H.; Hamid, S.B.A.; Su, D.S. Selective and Stable Ethylbenzene Dehydrogenation to Styrene over Nanodiamonds under Oxygen-lean Conditions. ChemSusChem 2016, 9, 662–669. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Truong-Phuoc, L.; Duong-Viet, C.; Nhut, J.-M.; Nguyen-Dinh, L.; Ersen, O.; Tuci, G.; Giambastiani, G.; Pham-Huu, C. N-Doped Food-Grade-Derived 3D Mesoporous Foams as Metal-Free Systems for Catalysis. ACS Catal. 2016, 6, 1408–1419. [Google Scholar] [CrossRef]
- Chizari, K.; Deneuve, A.; Ersen, O.; Florea, I.; Liu, Y.; Edouard, D.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-Doped Carbon Nanotubes as a Highly Active Metal-Free Catalyst for Selective Oxidation. ChemSusChem 2012, 5, 102–108. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Li, H.; Wang, Y. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- Luo, J.; Peng, F.; Wang, H.; Yu, H. Enhancing the catalytic activity of carbon nanotubes by nitrogen doping in the selective liquid phase oxidation of benzyl alcohol. Catal. Commun. 2013, 39, 44–49. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Yan, X.; Yao, X. Activity Origins in Nanocarbons for the Electrocatalytic Hydrogen Evolution Reaction. Small 2018, 14, e1800235. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, D.-W.; Amal, R.; Dai, L. Carbon-Based Metal-Free Catalysts for Key Reactions Involved in Energy Conversion and Storage. Adv. Mater. 2018, 31, e1801526. [Google Scholar] [CrossRef]
- Tuci, G.; Filippi, J.; Ba, H.; Rossin, A.; Luconi, L.; Pham-Huu, C.; Vizza, F.; Giambastiani, G. How to Teach an Old Dog New (Electrochemical) Tricks: Aziridine-Functionalized CNTs as Efficient Electrocatalysts for the Selective CO2 Reduction to CO. J. Mater. Chem. A 2018, 6, 16382–16389. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-Doped Graphene for Generation and Evolution of Reactive Radicals by Metal-Free Catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Ma, X.; Zhao, H.; Zhang, X. Facile Construction of Mesoporous N-Doped Carbons as Highly Efficient 4-Nitrophenol Reduction Catalysts. ChemCatChem 2015, 7, 3454–3459. [Google Scholar] [CrossRef]
- Liu, Y.; Duong-Viet, C.; Luo, J.; Hébraud, A.; Schlatter, G.; Ersen, O.; Nhut, J.-N.; Pham-Huu, C. One-Pot Synthesis of a Nitrogen-Doped Carbon Composite by Electrospinning as a Metal-Free Catalyst for Oxidation of H2S to Sulfur. ChemCatChem 2015, 7, 2957–2964. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Truong-Phuoc, L.; Duong-Viet, C.; Mu, X.; Doh, W.H.; Tran-Thanh, T.; Baaziz, W.; Nguyen-Dinh, L.; Nhut, J.-M.; et al. A highly N-doped carbon phase “dressing” of macroscopic supports for catalytic applications. Chem. Commun. 2015, 51, 14393–14396. [Google Scholar] [CrossRef] [Green Version]
- Duong-Viet, C.; Ba, H.; El-Berrichi, Z.; Nhut, J.-M.; Ledoux, M.J.; Liu, Y.; Pham-Huu, C. Silicon carbide foam as a porous support platform for catalytic applications. New J. Chem. 2016, 40, 4285–4299. [Google Scholar] [CrossRef]
- Sun, F.; Liu, J.; Chen, H.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Nitrogen-Rich Mesoporous Carbons: Highly Efficient, Regenerable Metal-Free Catalysts for Low-Temperature Oxidation of H2S. ACS Catal. 2013, 3, 862–870. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Truong-Phuoc, L.; Tran-Thanh, T.; Nhut, J.-M.; Nguyen-Dinh, L.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-doped carbon nanotubes decorated silicon carbide as a metal-free catalyst for partial oxidation of H2S. Appl. Catal. A Gen. 2014, 482, 397–406. [Google Scholar]
- Ba, H.; Duong-Viet, C.; Liu, Y.; Nhut, J.-M.; Granger, P.; Ledoux, M.J.; Pham-Huu, C. Nitrogen-doped carbon nanotube spheres as metal-free catalysts for the partial oxidation of H2S. C. R. Chim. 2016, 19, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wang, X.; Hou, Y.-N.; Pan, X.; Zhao, Z.; Qiu, J. Nitrogen-doped mesoporous carbon nanosheets derived from metal-organic frameworks in a molten salt medium for efficient desulfurization. Carbon 2017, 117, 376–382. [Google Scholar] [CrossRef]
- Shen, L.; Lei, G.; Fang, Y.; Cao, Y.; Wang, X.; Jiang, L. Polymeric carbon nitride nanomesh as an efficient and durable metal-free catalyst for oxidative desulfurization. Chem. Commun. 2018, 54, 2475–2478. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Ba, H.; Liu, Y.; Truong-Phuoc, L.; Nhut, J.M.; Pham-Huu, C. Nitrogen-doped carbon nanotubes on silicon carbide as a metal-free catalyst. Chin. J. Catal. 2014, 35, 906–913. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Giambastiani, G.; Liu, Y.; Ba, H.; Nguyen-Dinh, L.; Nhut, J.-M.; Duong-Viet, C. Method for preparing highly nitrogen-doped mesoporous carbon composites. EP Patent 3047905 A1, 29 April 2020. [Google Scholar]
- Mokhatab, S.; Poe, W.A. (Eds.) Typical [H2S] and [BTX] in sour gas are assumed close to 0.25 vol.% and about 2000 ppm, respectively, with the latter being tentatively composed by: Benzene = c.a. 900 ppm, Toluene = c.a. 750 ppm and Xylene = c.a. 400 ppm. In Handbook of Natural Gas Transmission and Processing; Gulf Professional Publishing: Houston, TX, USA, 2012. [Google Scholar]
- Duong-Viet, C.; Nhut, J.-M.; Truong-Huu, T.; Tuci, G.; Nguyen-Dinh, L.; Liu, Y.; Pham, C.; Giambastiani, G.; Pham-Huu, C. A Nitrogen-Doped Carbon Coated Silicon Carbide as a Robust and Highly Efficient Metal-Free Catalyst for Sour Gases Desulfurization in the Presence of Aromatics as Contaminants. Catal. Sci. Technol. 2020, 10, 5487–5500. [Google Scholar] [CrossRef]
- Mukasyan, A.S. Silicon Carbide. In Concise Encyclopedia of Self-Propagating High-Temperature Synthesis; Borovinskaya, I.P., Gromov, A.A., Levashov, E.A., Maksimov, Y.M., Mukasyan, A.S., Rogachev, A.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 336–338. [Google Scholar]
- Keller, N.; Pham-Huu, C.; Estournès, C.; Ledoux, M.J. Low temperature use of SiC-supported NiS2-based catalysts for selective H2S oxidation Role of SiC surface heterogeneity and nature of the active phase. Appl. Catal. A Gen. 2002, 234, 191–205. [Google Scholar] [CrossRef]
- Nguyen, P.; Edouard, D.; Nhut, J.M.; Ledoux, M.J.; Pham, C.; Pham-Huu, C. High thermal conductive b-SiC for selective oxidation of H2S: A new support for exothermal reactions. Appl. Catal. B Environ. 2007, 76, 300–310. [Google Scholar] [CrossRef]
- Terörde, R.J.A.M.; van den Brink, P.J.; Visser, L.M.; van Dillen, A.J.; Geus, J.W. Selective oxidation of hydrogen sulfide to elemental sulfur using iron oxide catalysts on various supports. Catal. Today 1993, 17, 217–224. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. Tuning the Acid/Base Properties of Nanocarbons by Functionalization via Amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef]
- Toluene was also Selected on the Basis of Its Intermediate Toxicity Degree among BTX Contaminants, i.e., Xylene > Toluene > Benzene. Available online: http://universulphur.com/mespon/2015_presentations/session_b/5.%20Dealing%20with%20Aromatics%20in%20the%20Sulfur%20Recovery%20Unit%20--%20Eric%20Roisin%20--%20Axens.pdf (accessed on 21 December 2020).
- Zhao, Z.; Dai, Y.; Ge, G.; Guo, X.; Wang, G. Facile simultaneous defect production and O,N-doping of carbon nanotubes with unexpected catalytic performance for clean and energy-saving production of styrene. Green Chem. 2015, 17, 3723–3727. [Google Scholar] [CrossRef]
- Jin, X.; Balasubramanian, V.V.; Selvan, S.T.; Sawant, D.P.; Chari, M.A.; Lu, G.Q.; Vinu, A. Highly Ordered Mesoporous Carbon Nitride Nanoparticles with High Nitrogen Content: A Metal-Free Basic Catalyst. Angew. Chem. Int. Ed. 2009, 48, 7884–7887. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. Catalytic Consequences of Spatial Constraints and Acid Site Location for Monomolecular Alkane Activation on Zeolites. J. Am. Chem. Soc. 2009, 131, 1958–1971. [Google Scholar] [CrossRef]
- Tuci, G.; Zafferoni, C.; Rossin, A.; Milella, A.; Luconi, L.; Innocenti, M.; Truong Phuoc, L.; Duong-Viet, C.; Pham-Huu, C.; Giambastiani, G. Chemically Functionalized Carbon Nanotubes with Pyridine Groups as Easily Tunable N-Decorated Nanomaterials for the Oxygen Reduction Reaction in Alkaline Medium. Chem. Mater. 2014, 26, 3460–3470. [Google Scholar] [CrossRef]
- Tuci, G.; Luconi, L.; Rossin, A.; Berretti, E.; Ba, H.; Innocenti, M.; Yakhvarov, D.; Caporali, S.; Pham-Huu, C.; Giambastiani, G. Aziridine-Functionalized Multiwalled Carbon Nanotubes: Robust and Versatile Catalysts for the Oxygen Reduction Reaction and Knoevenagel Condensation. ACS Appl. Mater. Interfaces 2016, 8, 30099–30106. [Google Scholar] [CrossRef]
- Moaseri, E.; Baniadam, M.; Maghrebi, M.; Karimi, M. A Simple Recoverable Titration Method for Quantitative Characterization of Amine-Functionalized Carbon Nanotubes. Chem. Phys. Lett. 2013, 555, 164–167. [Google Scholar] [CrossRef]
- Cai, Z.; Leong, E.; Wang, Z.; Niu, W.; Zhang, W.; Ravaine, S.; Yakovlev, N.; Liu, Y.; Teng, J.; Lu, X. Sandwich-structured Fe2O3@SiO2 @Au nanoparticles with magnetoplasmonic responses. J. Mater. Chem. C. 2015, 3, 11645–11652. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Fundamentals of Petroleum Refining. Elsevier, B.V.: Amsterdam, The Netherlands, 2010; pp. 377–402. [Google Scholar]
- Ebbing, D.D.; Gammon, S.D. General Chemistry, 9th ed.; Houghton Mifflin Company: Boston, MA, USA, 2009. [Google Scholar]





| Entry | Sample | SSA a (m2 g−1) | Total Pore Volume (cm3 g−1) b | Average Pore Size (nm) c | Surface Basic Sites (mmol g−1) d | N-C wt.% (from TGA) | N wt.% (from EA) | XPS Data, N-Species (%)f | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N at.% e | Pyridinic | Pyrrolic | Graphitic | Oxidized | ||||||||
| 1 | SiC | 30 | 0.21 | 27.3 | - g | - | - | - | - | - | - | - |
| 2 | N-C2/SiC | 69 | 0.11 | 14.8 | 0.63 | 6.7 | 2.1 | 5.1 | 36.8 | 26.0 | 25.6 | 11.6 |
| 3 | N-C4/SiC | 61 | 0.18 | 5.6 | 0.45 | 6.9 | 1.5 | 4.5 | 53.1 | 13.1 | 26.8 | 7.0 |
| 4 | Fe2O3/SiO2 | 160 | 0.40 | 10.3 | n.d. | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong-Viet, C.; Nhut, J.-M.; Truong-Huu, T.; Tuci, G.; Nguyen-Dinh, L.; Pham, C.; Giambastiani, G.; Pham-Huu, C. Tailoring Properties of Metal-Free Catalysts for the Highly Efficient Desulfurization of Sour Gases under Harsh Conditions. Catalysts 2021, 11, 226. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020226
Duong-Viet C, Nhut J-M, Truong-Huu T, Tuci G, Nguyen-Dinh L, Pham C, Giambastiani G, Pham-Huu C. Tailoring Properties of Metal-Free Catalysts for the Highly Efficient Desulfurization of Sour Gases under Harsh Conditions. Catalysts. 2021; 11(2):226. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020226
Chicago/Turabian StyleDuong-Viet, Cuong, Jean-Mario Nhut, Tri Truong-Huu, Giulia Tuci, Lam Nguyen-Dinh, Charlotte Pham, Giuliano Giambastiani, and Cuong Pham-Huu. 2021. "Tailoring Properties of Metal-Free Catalysts for the Highly Efficient Desulfurization of Sour Gases under Harsh Conditions" Catalysts 11, no. 2: 226. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020226