Electrodeposition of Fe-Complexes on Oxide Surfaces for Efficient OER Catalysis
Abstract
:1. Introduction
2. Results
2.1. Electrodeposition of Complexes 1 and 2
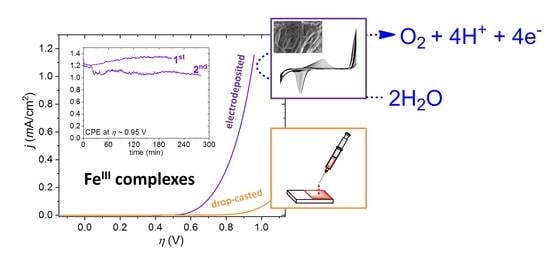
2.2. Performance of the Differently Fabricated, Immobilized Samples of 1 and 2 in Water Oxidation
2.3. Post-Catalytic Analysis of the Electrodeposited Samples by SEM-EDX
3. Materials and Methods
3.1. Electrochemistry in Non-Aqueous Solution
3.2. Drop-Casting and Dip-Coating
3.3. Electrodeposition of Complexes 1 and 2
3.4. Electrochemical Measurements in Aqueous Solution
3.5. Electrochemical Impedance Spectroscopy
3.6. Physical Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for Renewable Energy Production and Storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Mathew, S.; Hessels, J.; Reek, J.N.H.; Yu, F. Homogeneous Catalysts Based on First-Row Transition-Metals for Electrochemical Water Oxidation. ChemSusChem 2021, 14, 235–250. [Google Scholar] [CrossRef]
- Herrero, C.; Quaranta, A.; Leibl, W.; Rutherford, A.W.; Aukauloo, A. Artificial Photosynthetic Systems. Using Light and Water to Provide Electrons and Protons for the Synthesis of a Fuel. Energy Environ. Sci. 2011, 4, 2353–2365. [Google Scholar] [CrossRef]
- Yamazaki, H.; Shouji, A.; Kajita, M.; Yagi, M. Electrocatalytic and Photocatalytic Water Oxidation to Dioxygen Based on Metal Complexes. Coord. Chem. Rev. 2010, 254, 2483–2491. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Artificial Photosynthesis: Opportunities and Challenges of Molecular Catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [Green Version]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Wang, J.-W.; Hou, C.; Huang, H.-H.; Liu, W.-J.; Ke, Z.-F.; Lu, T.-B. Further Insight into the Electrocatalytic Water Oxidation by Macrocyclic Nickel(II) Complexes: The Influence of Steric Effect on Catalytic Activity. Catal. Sci. Technol. 2017, 7, 5585–5593. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Yu, F.; Shi, Y.; Li, F.; Li, H. Base-Enhanced Electrochemical Water Oxidation by a Nickel Complex in Neutral Aqueous Solution. Chem. Commun. 2019, 55, 6122–6125. [Google Scholar] [CrossRef]
- Lukács, D.; Szyrwiel, Ł.; Pap, J.S. Copper Containing Molecular Systems in Electrocatalytic Water Oxidation—Trends and Perspectives. Catalysts 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lei, Y.-J.; Xin, Z.-J.; Lu, Y.-B.; Wang, H.-Y. Water Splitting Based on Homogeneous Copper Molecular Catalysts. J. Photochem. Photobiol. A Chem. 2018, 355, 141–151. [Google Scholar] [CrossRef]
- Dogutan, D.K.; McGuire, R.; Nocera, D.G. Electocatalytic Water Oxidation by Cobalt(III) Hangman β-Octafluoro Corroles. J. Am. Chem. Soc. 2011, 133, 9178–9180. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Han, Y.; Lei, H.; Chen, M.; Cao, R. Cobalt Corroles with Phosphonic Acid Pendants as Catalysts for Oxygen and Hydrogen Evolution from Neutral Aqueous Solution. Chem. Commun. 2017, 53, 6195–6198. [Google Scholar] [CrossRef]
- Zhou, F.; Izgorodin, A.; Hocking, R.K.; Spiccia, L.; MacFarlane, D.R. Electrodeposited MnOx Films from Ionic Liquid for Electrocatalytic Water Oxidation. Adv. Energy Mater. 2012, 2, 1013–1021. [Google Scholar] [CrossRef]
- Hernández, S.; Ottone, C.; Varetti, S.; Fontana, M.; Pugliese, D.; Saracco, G.; Bonelli, B.; Armandi, M. Spin-Coated vs. Electrodeposited Mn Oxide Films as Water Oxidation Catalysts. Materials 2016, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Lee, Y.-M.; Hong, S.; Cho, K.-B.; Nishida, Y.; Seo, M.S.; Sarangi, R.; Fukuzumi, S.; Nam, W. Redox-Inactive Metal Ions Modulate the Reactivity and Oxygen Release of Mononuclear Non-Haem Iron(III)–Peroxo Complexes. Nat. Chem. 2014, 6, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (Hydro)Oxides for an Oxygen Evolution Electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yan, Z.; Chen, J. Electrodeposition Accelerates Metal-Based Batteries. Joule 2020, 4, 10–11. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Iwami, H.; Okamura, M.; Kondo, M.; Masaoka, S. Electrochemical Polymerization Provides a Function-Integrated System for Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 5965–5969. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Haigh, J. Strengths and Advantages of Electrodeposition as a Semiconductor Growth Technique for Applications in Macroelectronic Devices. J. Electrochem. Soc. 2005, 153, G47. [Google Scholar] [CrossRef]
- Roger, I.; Symes, M.D. First Row Transition Metal Catalysts for Solar-Driven Water Oxidation Produced by Electrodeposition. J. Mater. Chem. A 2016, 4, 6724–6741. [Google Scholar] [CrossRef] [Green Version]
- Váradi, T.; Pap, J.S.; Giorgi, M.; Párkányi, L.; Csay, T.; Speier, G.; Kaizer, J. Iron(III) Complexes with Meridional Ligands as Functional Models of Intradiol-Cleaving Catechol Dioxygenases. Inorg. Chem. 2013, 52, 1559–1569. [Google Scholar] [CrossRef]
- Al-Zuraiji, S.M.; Lukács, D.; Németh, M.; Frey, K.; Benkó, T.; Illés, L.; Pap, J.S. An Iron(III) Complex with Pincer Ligand—Catalytic Water Oxidation through Controllable Ligand Exchange. Reactions 2020, 1, 16–36. [Google Scholar] [CrossRef]
- Kripli, B.; Baráth, G.; Balogh-Hergovich, É.; Giorgi, M.; Simaan, A.J.; Párkányi, L.; Pap, J.S.; Kaizer, J.; Speier, G. Correlation between the SOD-like Activity of Hexacoordinate Iron(II) Complexes and Their Fe3+/Fe2+ Redox Potentials. Inorg. Chem. Commun. 2011, 14, 205–209. [Google Scholar] [CrossRef]
- Martić, G.; Engle, J.T.; Ziegler, C.J. Complexes of 1,3-Bis(2-Thiazolylimino)Isoindoline with Middle and Late First Row Transition Metals. Inorg. Chem. Commun. 2011, 14, 1749–1752. [Google Scholar] [CrossRef]
- Meder, M.B.; Gade, L.H. Coordination Chemistry of 1,3-Bis(2-Pyridylimino)- and 1,3-Bis(2-Thiazolylimino)Soindole Copper Complexes: Investigation of Their Catalytic Behavior in Oxidation Reactions. Eur. J. Inorg. Chem. 2004, 2004, 2716–2722. [Google Scholar] [CrossRef]
- Al-Zuraiji, S.M.; Benkó, T.; Illés, L.; Németh, M.; Frey, K.; Sulyok, A.; Pap, J.S. Utilization of Hydrophobic Ligands for Water-Insoluble Fe(II) Water Oxidation Catalysts—Immobilization and Characterization. J. Catal. 2020, 381, 615–625. [Google Scholar] [CrossRef]
- Csonka, R.; Speier, G.; Kaizer, J. Isoindoline-Derived Ligands and Applications. RSC Adv. 2015, 5, 18401–18419. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukács, D.; Németh, M.; Szyrwiel, Ł.; Illés, L.; Pécz, B.; Shen, S.; Pap, J.S. Behavior of a Cu-Peptide Complex under Water Oxidation Conditions—Molecular Electrocatalyst or Precursor to Nanostructured CuO Films? Sol. Energy Mater. Sol. Cells 2019, 201, 110079. [Google Scholar] [CrossRef]
- Beltrán-Suito, R.; Forstner, V.; Hausmann, J.N.; Mebs, S.; Schmidt, J.; Zaharieva, I.; Laun, K.; Zebger, I.; Dau, H.; Menezes, P.W.; et al. A Soft Molecular 2Fe–2As Precursor Approach to the Synthesis of Nanostructured FeAs for Efficient Electrocatalytic Water Oxidation. Chem. Sci. 2020, 11, 11834–11842. [Google Scholar] [CrossRef]







| Sample | Tafel Slope (mV/dec) | R1 (Ω) | R2 (Ω) | P (μFs(1−n)) a | nb |
|---|---|---|---|---|---|
| 1-DC-Nf@ITO (0.02 μmol) | 102.4(8) | 61.6(13) | 291(13) × 103 | 19.9(4) | 0.947(5) |
| 1-DC-Nf@ITO (0.04 μmol) | 91.1(9) | 96.4(8) | 151(4) × 103 | 19.3(3) | 0.952(5) |
| 1-DIP-Nf@ITO | 68.5(6) | 121.1(18) | 51(2) × 103 | 16.0(4) | 0.909(5) |
| 1-ED@FTO | 58.5(4) | 242.5(3) | 259.5(8) | 122.7(6) | 0.691(8) |
| 2-ED@FTO | 61.4(3) | 211.7(3) | 426.5(12) | 86.5(3) | 0.676(7) |
| 2-DC-Nf@ITO (0.04 μmol) | 130.2(2) | 388.6(7) | 53(9) × 103 | 21.3(2) | 0.964(10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zuraiji, S.M.; Benkó, T.; Frey, K.; Kerner, Z.; Pap, J.S. Electrodeposition of Fe-Complexes on Oxide Surfaces for Efficient OER Catalysis. Catalysts 2021, 11, 577. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050577
Al-Zuraiji SM, Benkó T, Frey K, Kerner Z, Pap JS. Electrodeposition of Fe-Complexes on Oxide Surfaces for Efficient OER Catalysis. Catalysts. 2021; 11(5):577. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050577
Chicago/Turabian StyleAl-Zuraiji, Sahir M., Tímea Benkó, Krisztina Frey, Zsolt Kerner, and József S. Pap. 2021. "Electrodeposition of Fe-Complexes on Oxide Surfaces for Efficient OER Catalysis" Catalysts 11, no. 5: 577. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050577