Ex-LDH-Based Catalysts for CO2 Conversion to Methanol and Dimethyl Ether
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of As-Prepared Ex-LDH Catalysts
2.2. Characterization of the H2-Treated Ex-LDH Catalysts
2.3. Characterization of the Zeolite Catalysts
2.4. Catalytic Results for CO2 Conversion to Methanol
2.5. Catalytic Results for CO2 Conversion to Dimethyl Ether
3. Materials and Methods
3.1. Materials
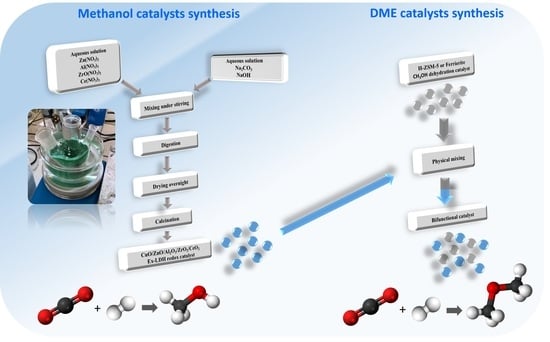
3.2. Preparation of Catalysts
3.2.1. Preparation of Hydrogenation Catalysts for Methanol Synthesis
3.2.2. Preparation of Bifunctional Catalysts for DME Synthesis
3.3. Characterization of Catalysts
3.4. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.; Kwon, S.; Song, Y.; Na, K. Catalytic CO2 hydrogenation using mesoporous bimetallic spinel oxides as active heterogeneous base catalysts with long lifetime. J. CO2 Util. 2020, 36, 145–152. [Google Scholar] [CrossRef]
- Bellotti, D.; Sorce, A.; Rivarolo, M.; Magistri, L. Techno-economic analysis for the integration of a power to fuel system with a CCS coal power plant. J. CO2 Util. 2019, 33, 262–272. [Google Scholar] [CrossRef]
- Panzone, C.; Philippe, R.; Chappaz, A.; Fongarland, P.; Bengaouer, A. Power-to-Liquid catalytic CO2 valorization into fuels and chemicals: Focus on the Fisher-Tropsch route. J. CO2 Util. 2020, 38, 314–347. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, C.; Chen, L.; Zhang, Y.; Fu, M.; Wu, J.; Ye, D. Active site structure study of Cu/Plate ZnO model catalysts for CO2 hydrogenation to methanol under the real reaction conditions. J. CO2 Util. 2020, 37, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; Cao, C.; Tian, P.; Zhu, M.; Zhang, Y.; Xu, J.; Tian, Y.; Han, Y.F. Resolving CO2 activation and hydrogenation pathways over iron carbides from DFT investigation. J. CO2 Util. 2020, 38, 10–15. [Google Scholar] [CrossRef]
- Youn, M.H.; Park, K.T.; Lee, Y.H.; Kang, S.P.; Lee, S.M.; Kim, S.S.; Kim, Y.E.; Ko, Y.N.; Jeong, S.K.; Lee, W. Carbon dioxide sequestration process for the cement industry. J. CO2 Util. 2019, 34, 325–334. [Google Scholar] [CrossRef]
- Runge, P.; Sölch, C.; Albert, J.; Wasserscheid, P.; Zöttl, G.; Grimm, V. Economic comparison of different electric fuels for energy scenarios in 2035. Appl. Energy 2019, 223–224, 1078–1093. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, H.; Gao, P.; Li, X.; Zhang, J.; Liu, H.; Wang, H.; Wei, W.; Sun, Y. Slurry methanol synthesis from CO2 hydrogenation over micro-spherical SiO2 support Cu/ZnO catalysts. J. CO2 Util. 2018, 26, 642–651. [Google Scholar] [CrossRef]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Chen, J.G. CO2 Hydrogenation to Methanol over ZrO2-Containing Catalysts: Insights into ZrO2 Induced Synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Kousi, K.; Kondarides, D.I. CO2 Hydrogenation to Methanol over La2O3-Promoted CuO/ZnO/Al2O3 Catalysts: A Kinetic and Mechanistic Study. Catalysts 2020, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Mota, N.; Millán Ordoñez, E.; Pawelwc, B.; Fierro, J.L.G.; Navarro, R.M. Direct Synthesis of Dimethyl Ether from CO2: Recent Advances in Bifunctional/Hybrid Catalytic Systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- Gao, W.; Wang, H.; Wang, Y.; Guo, W.; Jia, M. Dimethyl ether synthesis from CO2 hydrogenation on La-modified CuO-ZnO-Al2O3/HZSM-5 bifunctional catalysts. J. Rare Earths 2013, 31, 470–476. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Mezzapica, A.; Spadaro, L.; Arena, F.; Frusteri, F. Catalytic behaviour of a bifunctional system for the one step synthesis of DME by CO2 hydrogenation. Catal. Today 2014, 228, 51–57. [Google Scholar] [CrossRef]
- Montesano, R.; Narvaez, A.; Chadwick, D. Shape-selectivity effects in syngas-to-dimethyl ether conversion over Cu/ZnO/Al2O3 and zeolite mixtures: Carbon deposition and by-product formation. Appl. Catal. A Gen. 2014, 482, 69–77. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogen reaction: New evidences of a superior behaviour of FER-based hybrid systems to obtain high DME. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Micropor. Mesopor. Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Ren, S.; Shoemaker, W.R.; Wang, X.; Shang, Z.; Klinghoffer, N.; Li, S.; Yu, M.; He, X.; White, T.A.; Liang, X. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Ren, S.; Li, S.; Klinghoffer, N.; Yu, M.; Liang, X. Effects of mixing methods of bifunctional catalysts on catalyst stability of DME synthesis via CO2 hydrogenation. Carbon Resour. Convers. 2019, 2, 85–94. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Mezzapica, A.; Frusteri, F. DME production by CO2 hydrogenation: Key factors affecting the behaviour of CuZnZr/ferrierite catalysts. Catal. Today 2017, 281, 337–344. [Google Scholar] [CrossRef]
- Sheng, Q.; Ye, R.-P.; Gong, W.; Shi, X.; Xu, B.; Argyle, M.; Adidharma, H.; Fan, M. Mechanism and catalytic performance for direct dimethyl ether synthesis by CO2 hydrogenation over CuZnZr/ferrierite hybrid catalyst. J. Environ. Sci. 2020, 92, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Tan, Y.; Xie, H.; Zhang, Q.; Han, Y. Direct synthesis of dimethyl ether from biomass-derived syngas over Cu-ZnO-Al2O3-ZrO2(x)/γ-Al2O3 bifunctional catalysts: Effect of Zr-loading. Fuel Process. Technol. 2014, 126, 88–94. [Google Scholar] [CrossRef]
- Suwannapichat, Y.; Numpilai, T.; Chanlek, N.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Direct synthesis of dimethyl ether from CO2 hydrogenation over novel hybrid catalysts containing a Cu–ZnO–ZrO2 catalyst admixed with WOx/Al2O3 catalysts: Effects of pore size of Al2O3 support and W loading content. Energy Convers. Manag. 2018, 159, 20–29. [Google Scholar] [CrossRef]
- Muhamad, E.N.; Irmawati, R.; Tautiq-Yap, Y.H.; Abdullah, A.H.; Kniep, B.L.; Girgsdies, F.; Ressler, T. Comparative study of Cu/ZnO catalysts derived from different precursors as a function of aging. Catal. Today 2008, 131, 118–124. [Google Scholar] [CrossRef]
- Baltes, C.; Vukojevic, S.; Schuth, F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, Y.Z.; Han, M.H.; Wang, J.F.; Jin, Y.; Wei, F. Long carbon nanotubes intercrossed Cu/Zn/Al/Zr catalyst for CO/CO2 hydrogenation to methanol/dimethyl ether. Catal. Today 2010, 150, 55–60. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Xiao, F.; Zhao, N.; Sun, N.; Wei, W.; Zhong, L.; Sun, Y. Preparation and activity of Cu/Zn/Al/Zr catalysts via hydrotalcite-containing precursors for methanol synthesis from CO2 hydrogenation. Catal. Sci. Technol. 2012, 2, 1447–1454. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Todaro, S.; Frusteri, F.; Bonura, G. Tailoring of Hydrotalcite-Derived Cu-Based Catalysts for CO2 Hydrogenation to Methanol. Catalysts 2019, 9, 1058. [Google Scholar] [CrossRef] [Green Version]
- Kühl, S.; Schumann, J.; Kasatkin, I.; Havecker, M.; Schlögl, R.; Behrens, M. Ternary and quaternary Cr or Ga-containing ex-LDH catalysts—Influence of the additional oxides onto the microstructure and activity of Cu/ZnAl2O4 catalysts. Catal. Today 2015, 246, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Kasatkin, I.; Kühl, S.; Weinberg, G. Phase-Pure Cu,Zn,Al Hydrotalcite-like Materials as Precursors for Copper rich Cu/ZnO/Al2O3 Catalysts. Chem. Mater. 2010, 22, 386–397. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press-Elsevier: Amsterdam, The Netherlands, 2014; pp. 12–13. [Google Scholar]
- Guo, X.; Mao, D.; Wang, S.; Wu, G.; Lu, G. Combustion synthesis of CuO-ZnO-ZrO2 catalysts for the hydrogenation of carbon dioxide to methanol. Catal. Commun. 2009, 10, 1661–1664. [Google Scholar] [CrossRef]
- Jang, J.; Kim, G.-P.; Chang, T.-S.; Kim, B.-S.; Shim, S.F.; Park, S.H.; Baeck, S.-H. Preparation of Nanostructured CuO/ZnO/Al2O3 Catalysts for the Synthesis of Methanol from Syngas. J. Nanosci. Nanotechnol. 2016, 16, 10887–10891. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Fluorine-modified Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Commun. 2014, 50, 78–82. [Google Scholar] [CrossRef]
- Gao, P.; Xie, R.; Wang, H.; Zhong, L.; Xia, L.; Zhang, Z.; Wei, W.; Sun, Y. Cu/Zn/Al/Zr catalysts via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide. J. CO2 Util. 2015, 11, 41–48. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Gao, P.; Zhong, L.; Li, X.; Zhang, Z.; Wang, H.; Wei, W.; Sun, Y. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Today 2017, 281, 327–336. [Google Scholar] [CrossRef]
- Jung, K.-D.; Joo, O.-S.; Han, S.-H. Structural change of Cu/ZnO by reduction of ZnO in Cu/ZnO with methanol. Catal. Lett. 2000, 68, 49–54. [Google Scholar] [CrossRef]
- Hajduk, Š.; Dasireddy, V.D.B.C.; Likozar, B.; Dražić, G.; Orel, Z.C. COx-free hydrogen production via decomposition of ammonia over Cu–Zn-based heterogeneous catalysts and their activity/stability. Appl. Catal. B Environ. 2017, 211, 57–67. [Google Scholar] [CrossRef]
- Atzori, L.; Rombi, E.; Meloni, D.; Monaci, R.; Sini, M.F.; Cutrufello, M.G. Nanostructured Ni/CeO2-ZrO2 catalysts for CO2 conversion into SNG. J. Nanosci. Nanotechnol. 2019, 19, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Fujimoto, K. Development of highly stable catalyst for methanol synthesis from carbon dioxide. Appl. Catal. A Gen. 2014, 469, 306–311. [Google Scholar] [CrossRef]
- Bonura, G.; Arena, F.; Mezzatesta, G.; Canilla, C.; Spadaro, L.; Frusteri, F. Role of the ceria promoter and carrier on the functionality of Cu-based catalysts in the CO2-to-methanol hydrogenation reaction. Catal. Today 2011, 171, 251–256. [Google Scholar] [CrossRef]
- Angelo, L.; Kobl, K.; Martinez Tejada, L.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.-C. Study of CuZnMOx oxides (M = Al, Zr, Ce, CeZr) for the catalytic hydrogenation of CO2 into methanol. C. R. Chim. 2015, 18, 250–260. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2013, 468, 442–452. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Wu, D. Ternary copper-cerium-zirconium mixed metal oxide catalyst for direct CO2 hydrogenation to methanol. Mater. Chem. Phys. 2018, 219, 263–272. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. An investigation of Zr/Ce ratio influencing the catalytic performance of CuO/Ce1-xZrxO2 catalyst for CO2 hydrogenation to CH3OH. J. Energy Chem. 2020, 47, 18–28. [Google Scholar] [CrossRef]
- García-Trenco, A.; Martínez, A. Direct synthesis of DME from syngas on hybrid CuZnAl/ZSM-5 catalysts: New insights into the role of zeolite acidity. Appl. Catal. A Gen. 2012, 411–412, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Bonura, G.; Cordaro, M.; Spadaro, L.; Cannilla, C.; Arena, F.; Frusteri, F. Hybrid Cu-ZnO-ZrO2/H-ZSM5 system for the direct synthesis of DME by CO2 hydrogenation. Appl. Catal. B Environ. 2013, 140–141, 16–24. [Google Scholar] [CrossRef]
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley & Sons Inc.: New York, NY, USA, 1974; pp. 687–703. [Google Scholar]
- Gervasini, A.; Bennici, S. Dispersion and surface states of copper catalysts by temperature-programmed-reduction of oxidized surfaces (s-TPR). Appl. Catal. A Gen. 2005, 281, 199–205. [Google Scholar] [CrossRef]
- Pettinau, A.; Mureddu, M.; Ferrara, F. Carbon Dioxide Conversion into Liquid Fuels by Hydrogenation and Photoelectrochemical Reduction: Project Description and Preliminary Experimental Results. Energy Procedia 2017, 114, 6893–6904. [Google Scholar] [CrossRef]
- Mureddu, M.; Ferrara, F.; Pettinau, A. Highly efficient CuO/ZnO/ZrO2@SBA-15 nanocatalysts for methanol synthesis from the catalytic hydrogenation of CO2. Appl. Catal. B Environ. 2019, 258, 117941. [Google Scholar] [CrossRef]





| Sample | Molar Ratio | Composition (wt%) | ||||
|---|---|---|---|---|---|---|
| Cu/Zn/Al/Zr/Ce | CuO | ZnO | Al2O3 | ZrO2 | CeO2 | |
| CuZnAl | 2.1/1.0/0.98 | 56 | 27 | 17 | - | - |
| (nominal: 2/1/1) | ||||||
| CuZnAlZr | 2.0/1.0/0.80/0.24 | 51 | 26 | 13 | 10 | - |
| (nominal: 2/1/0.7/0.3) | ||||||
| CuZnAlCe | 2.0/1.0/0.76/0.31 | 48 | 24 | 12 | - | 16 |
| (nominal: 2/1/0.7/0.3) | ||||||
| CuZnAlZrCe | 2.0/1.0/0.76/0.13/0.16 | 49 | 25 | 12 | 5 | 9 |
| (nominal: 2/1/0.7/0.15/0.15) | ||||||
| Sample | SBET (m2 g−1) | Vp (cm3 g−1) |
|---|---|---|
| CuZnAl | 45 | 0.17 |
| CuZnAlZr | 64 | 0.38 |
| CuZnAlCe | 49 | 0.39 |
| CuZnAlZrCe | 66 | 0.41 |
| Sample | nA (μmol g−1) | nB (μmol g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| nA,w (a) | nA,m (b) | nA,s (c) | nA,tot (d) | nB,w (e) | nB,m (f) | nB,s (g) | nB,tot (h) | |
| CuZnAl | 23 | 15 | 15 | 53 | 0.2 | 0.2 | 1.8 | 2.2 |
| CuZnAlZr | 38 | 13 | 19 | 70 | 10 | 6 | - | 16 |
| CuZnAlCe | 47 | 17 | 20 | 84 | 19 | 10 | - | 29 |
| CuZnAlZrCe | 47 | 6 | 33 | 86 | 16 | 9 | - | 25 |
| Sample | Cu Content (a) (wt%) | DCu0 (%) | (m2 g−1) |
|---|---|---|---|
| CuZnAl | 45 | 6.4 | 19 |
| CuZnAlZr | 41 | 6.8 | 18 |
| CuZnAlCe | 38 | 8.1 | 20 |
| CuZnAlZrCe | 39 | 8.0 | 20 |
| Sample | nA (μmol g−1) | nB (μmol g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| nA,w (a) | nA,m (b) | nA,s (c) | nA,tot (d) | nB,w (e) | nB,m (f) | nB,s (g) | nB,tot (h) | |
| CuZnAl | 53 | 17 | 12 | 82 | 3 | 1 | 3 | 7 |
| CuZnAlZr | 67 | 23 | 19 | 109 | 18 | 3 | 8 | 29 |
| CuZnAlCe | 102 | 29 | 12 | 143 | 20 | 13 | 16 | 49 |
| CuZnAlZrCe | 72 | 20 | 17 | 109 | 14 | 6 | 14 | 34 |
| Sample | nA (μmol g−1) | nA Distribution (%) | |||||
|---|---|---|---|---|---|---|---|
| nA,w (a) | nA,m (b) | nA,s (c) | nA,tot (d) | nA,w/nA,tot | nA,m/nA,tot | nA,s/nA,tot | |
| FER_20 | 1292 | 681 | 83 | 2056 | 63 | 33 | 4 |
| ZSM5_23 | 1293 | 555 | 56 | 1904 | 68 | 29 | 3 |
| ZSM_350 | 96 | 45 | 33 | 174 | 55 | 26 | 19 |
| Catalyst | XCO2 (mol%) | SCO (mol%) | SCH3OH (mol%) | SDME (mol%) | SCnH(2n+2) (mol%) | YCH3OH (mol%) | STYCH3OH (mgCH3OH gcat−1 h−1) |
|---|---|---|---|---|---|---|---|
| CuZnAl | 18.6 | 65.3 | 27.7 | 6.9 | 0.1 | 5.2 | 205 |
| CuZnAlZr | 18.7 | 66.3 | 33.7 | traces | traces | 6.3 | 250 |
| CuZnAlCe | 14.2 | 61.6 | 37.8 | 0.6 | traces | 5.4 | 213 |
| CuZnAlZrCe | 17.9 | 64.7 | 32.5 | 2.6 | 0.2 | 5.8 | 230 |
| Catalyst | XCO2 (mol%) | SCO (mol%) | SCH3OH (mol%) | SDME (mol%) | SCnH(2n+2) (mol%) | YDME (mol%) | STYDME (mgDME gcat−1 h−1) |
|---|---|---|---|---|---|---|---|
| CuZnAl/FER_20 | 17.3 | 61.4 | 10.5 | 28.1 | - | 4.9 | 139 |
| CuZnAlZr/FER_20 | 17.0 | 58.7 | 9.1 | 32.0 | 0.2 | 5.4 | 161 |
| CuZnAlCe/FER_20 | 13.4 | 63.0 | 10.6 | 26.4 | traces | 3.5 | 100 |
| CuZnAlZrCe/FER_20 | 15.8 | 62.4 | 9.9 | 27.6 | 0.1 | 4.4 | 124 |
| CuZnAlZr/ZSM5_23 | 15.4 | 59.0 | 9.1 | 31.8 | 0.1 | 4.9 | 140 |
| CuZnAlZr/ZSM5_350 | 18.9 | 56.9 | 9.9 | 33.2 | traces | 6.3 | 179 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureddu, M.; Lai, S.; Atzori, L.; Rombi, E.; Ferrara, F.; Pettinau, A.; Cutrufello, M.G. Ex-LDH-Based Catalysts for CO2 Conversion to Methanol and Dimethyl Ether. Catalysts 2021, 11, 615. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050615
Mureddu M, Lai S, Atzori L, Rombi E, Ferrara F, Pettinau A, Cutrufello MG. Ex-LDH-Based Catalysts for CO2 Conversion to Methanol and Dimethyl Ether. Catalysts. 2021; 11(5):615. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050615
Chicago/Turabian StyleMureddu, Mauro, Sarah Lai, Luciano Atzori, Elisabetta Rombi, Francesca Ferrara, Alberto Pettinau, and Maria Giorgia Cutrufello. 2021. "Ex-LDH-Based Catalysts for CO2 Conversion to Methanol and Dimethyl Ether" Catalysts 11, no. 5: 615. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050615