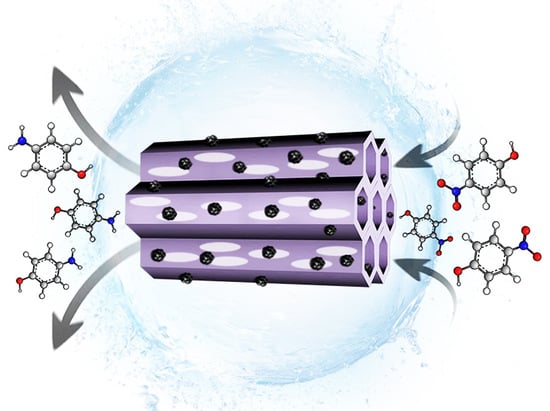
The Isocyanurate-Carbamate-Bridged Hybrid Mesoporous Organosilica: An Exceptional Anchor for Pd Nanoparticles and a Unique Catalyst for Nitroaromatics Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and the Characterisation of the Catalyst
2.2. Catalytic Test
3. Materials and Methods
3.1. Materials Characterisations
3.2. Materials Synthesis
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A. Mesoporous sieves with unified hybrid inorganic/organic frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Hasani, M.; Ide, Y.; Rostamnia, S.; Tsunoji, N.; Hussein, N.; Assadi, M. Merging periodic mesoporous organosilica (PMO) with mesoporous aluminosilica (Al/Si-PMO): A catalyst for green oxidation. Mol. Catal. 2020, 482, 110676. [Google Scholar] [CrossRef]
- Doustkhah, E.; Tahawy, R.; Simon, U.; Tsunoji, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. Bispropylurea bridged polysilsesquioxane: A microporous MOF-like material for molecular recognition. Chemosphere 2021, 276, 130181. [Google Scholar] [CrossRef] [PubMed]
- Esmat, M.; Mohtasham, H.; GadelHak, Y.; Mehrebani, R.T.; Tahawy, R.; Rostamnia, S.; Fukata, N.; Khaksar, S.; Doustkhah, E.J.C. 2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction. Catalysts 2020, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Elhamifar, D.; Yari, O.; Karimi, B. Highly ordered mesoporous organosilica–titania with ionic liquid framework as very efficient nanocatalyst for green oxidation of alcohols. J. Colloid Interface Sci. 2017, 500, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, K.Z.; Yamini, Y.; Karimi, B.; Seidi, S.; Khorasani, M.; Ghaemmaghami, M.; Vali, H. Imidazolium-based mesoporous organosilicas with bridging organic groups for microextraction by packed sorbent of phenoxy acid herbicides, polycyclic aromatic hydrocarbons and chlorophenols. Microchim. Acta 2019, 186, 239. [Google Scholar] [CrossRef]
- Chandra, D.; Das, S.K.; Bhaumik, A. A fluorophore grafted 2D-hexagonal mesoporous organosilica: Excellent ion-exchanger for the removal of heavy metal ions from wastewater. Microporous Mesoporous Mater. 2010, 128, 34–40. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Advanced molecular recognition of 3-nitro-L-tyrosine: The use of zwitterion embedded molecularly imprinted mesoporous organosilica with sub-nanomolar sensitivity. Biosens. Bioelectron. 2020, 160, 112216. [Google Scholar] [CrossRef]
- Yamanaka, K.-I.; Maegawa, Y.; Yamada, Y.; Inagaki, S. Excited-State Dynamics of 2,2′-Bipyridine Moieties Embedded in the Framework of Periodic Mesoporous Organosilica. J. Phys. Chem. C 2019, 123, 28443–28449. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Maegawa, Y.; Abalymov, A.; Skirtach, A.G.; Inagaki, S.; Van Der Voort, P. Lanthanide-Grafted Bipyridine Periodic Mesoporous Organosilicas (BPy-PMOs) for Physiological Range and Wide Temperature Range Luminescence Thermometry. ACS Appl. Mater. Interfaces 2020, 12, 13540–13550. [Google Scholar] [CrossRef] [PubMed]
- Etienne, M.; Goux, A.; Sibottier, E.; Walcarius, A.J. Oriented mesoporous organosilica films on electrode: A new class of nanomaterials for sensing. J. Nanosci. Nanotechnol. 2009, 9, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, H.; Yoshida, M.; Tsunenari, T.; Nozawa, S.; Sato-Tomita, A.; Maegawa, Y.; Inagaki, S.; Kobayashi, A.; Kato, M. Fast and stable vapochromic response induced through nanocrystal formation of a luminescent platinum(II) complex on periodic mesoporous organosilica. Sci. Rep. 2019, 9, 15151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Li, X.; Zhang, R.; Liu, Y.; Wang, W.; Ling, Y.; El-Toni, A.M.; Zhao, D. Periodic Mesoporous Organosilica Nanocubes with Ultrahigh Surface Areas for Efficient CO2 Adsorption. Sci. Rep. 2016, 6, 20769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebardasti, A.; Dekamin, M.G.; Doustkhah, E.; Assadi, M.H.N. Carbamate-Isocyanurate-Bridged Periodic Mesoporous Organosilica for van der Waals CO2 Capture. Inorg. Chem. 2020, 59, 11223–11227. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Mohtasham, H.; Farajzadeh, M.; Rostamnia, S.; Wang, Y.; Arandiyan, H.; Assadi, N.H.M. Organosiloxane tunability in mesoporous organosilica and punctuated Pd nanoparticles growth; theory and experiment. Microporous Mesoporous Mater. 2020, 293, 109832. [Google Scholar] [CrossRef]
- Hunks, W.J.; Ozin, G.A. Challenges and advances in the chemistry of periodic mesoporous organosilicas (PMOs). J. Mater. Chem. 2005, 15, 3716–3724. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hassandoost, R.; Khataee, A.; Luque, R.; Assadi, M.H.N. Hard-templated metal–organic frameworks for advanced applications. Chem. Soc. Rev. 2021, 50, 2927–2953. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Rostamnia, S.; Imura, M.; Ide, Y.; Mohammadi, S.; Hyland, C.J.T.; You, J.; Tsunoji, N.; Zeynizadeh, B.; Yamauchi, Y. Thiourea bridged periodic mesoporous organosilica with ultra-small Pd nanoparticles for coupling reactions. RSC Adv. 2017, 7, 56306–56310. [Google Scholar] [CrossRef] [Green Version]
- Doustkhah, E.; Rostamnia, S.; Zeynizadeh, B.; Kim, J.; Yamauchi, Y.; Ide, Y. Efficient H2 Generation Using Thiourea-based Periodic Mesoporous Organosilica with Pd Nanoparticles. Chem. Lett. 2018, 47, 1243–1245. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Bulgar, R.; Zeynizadeh, B. Supported palladium ions inside periodic mesoporous organosilica with ionic liquid framework (Pd@IL-PMO) as an efficient green catalyst for S-arylation coupling. Microporous Mesoporous Mater. 2016, 225, 272–279. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Arefi, E.; Yaghoubi, A. Isocyanurate-based periodic mesoporous organosilica (MO-ICS): A highly efficient and recoverable nanocatalyst for the one-pot synthesis of substituted imidazoles and benzimidazoles. RSC Adv. 2016, 6, 86982–86988. [Google Scholar] [CrossRef]
- Haghighat, M.; Shirini, F.; Golshekan, M. Synthesis of tetrahydrobenzo[b]pyran and Pyrano [2, 3-d]pyrimidinone derivatives using Fe3O4@Ph-MO-NaHSO4 as a new magnetically separable nanocatalyst. J. Nanosci. Nanotechnol. 2019, 19, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, A.; Dekamin, M.G.; Arefi, E.; Karimi, B. Propylsulfonic acid-anchored isocyanurate-based periodic mesoporous organosilica (PMO-ICS-Pr-SO3H): A new and highly efficient recoverable nanoporous catalyst for the one-pot synthesis of bis(indolyl)methane derivatives. J. Colloid Interface Sci. 2017, 505, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ma, J.; Wang, J.; Jiang, W.; Zhang, W.-X.; Yang, J. Site-selective exposure of iron nanoparticles to achieve rapid interface enrichment for heavy metals. Chem. Commun. 2020, 56, 2795–2798. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Mizoshita, N.; Ohsuna, T.; Tani, T.; Inagaki, S. Crystal-like periodic mesoporous organosilica bearing pyridine units within the framework. Chem. Commun. 2010, 46, 8163–8165. [Google Scholar] [CrossRef]
- Zou, H.; Wang, R.; Dai, J.; Wang, Y.; Wang, X.; Zhang, Z.; Qiu, S. Amphiphilic hollow porous shell encapsulated Au@Pd bimetal nanoparticles for aerobic oxidation of alcohols in water. Chem. Commun. 2015, 51, 14601–14604. [Google Scholar] [CrossRef]
- Attia, M.F.; Swasy, M.I.; Ateia, M.; Alexis, F.; Whitehead, D.C. Periodic mesoporous organosilica nanomaterials for rapid capture of VOCs. Chem. Commun. 2020, 56, 607–610. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Nanoporous silica-supported organocatalyst: A heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 2014, 4, 28238–28248. [Google Scholar] [CrossRef]
- Esquivel, D.; Amaro-Gahete, J.; Caballero-Casero, N.; Jiménez-Sanchidrián, C.; Ruiz, J.R.; Rubio, S.; Van Der Voort, P.; Romero-Salguero, F.J. Tailoring Bifunctional Periodic Mesoporous Organosilicas for Cooperative Catalysis. ACS Appl. Nano Mater. 2020, 3, 2373–2382. [Google Scholar] [CrossRef]
- Zarei, M.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Noroozizadeh, E.; Rostamnia, S. Three-Component Synthesis of Spiropyrans Using SBA-15/En Bonded Phosphorous Acid [SBA-15/Pr-NH1-y(CH2PO3H2)y-Et-NH2−x(CH2PO3H2)x] as a New Nanoporous Heterogeneous Catalyst. ChemistrySelect 2018, 3, 12144–12149. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Narani, A.; Reddy Kannapu, H.P.; Natte, K.; Burri, D.R. Pd-Nanoparticles immobilized organo-functionalized SBA-15: An efficient heterogeneous catalyst for selective hydrogenation of CC double bonds of α,β-unsaturated carbonyl compounds. Mol. Catal. 2020, 497, 111200. [Google Scholar] [CrossRef]
- Yu, Y.; Li, F.; Zang, Z.; Xu, L.; Liu, G. Highly efficient selective oxidation of 2-methylnaphthalene to vitamin K3 over mesoporous Al/Ti-SBA-15 catalysts: The effect of acid sites and textural property. Mol. Catal. 2020, 495, 111158. [Google Scholar] [CrossRef]
- Yang, X.; Niu, L.; Xia, Z.; Yan, X.; Bai, G. Preparation of Ni/mSiO2 with the existence of hydrogelator: Insight into hydrogelator self-assembly on metal dispersion and catalytic performance in quinoline hydrogenation. Mol. Catal. 2020, 493, 111094. [Google Scholar] [CrossRef]
- Szabó, B.; Novodárszki, G.; May, Z.; Valyon, J.; Hancsók, J.; Barthos, R. Conversion of ethanol to butadiene over mesoporous In2O3-promoted MgO-SiO2 catalysts. Mol. Catal. 2020, 491, 110984. [Google Scholar] [CrossRef]
- Shimizu, M.; Michikawa, K.; Maegawa, Y.; Inagaki, S.; Fujita, K.-I. Iridium Complex Immobilized on Custom-Designed Periodic Mesoporous Organosilica as Reusable Catalyst for the Dehydrogenative Oxidation of Alcohols. Acs Appl. Nano Mater. 2020, 3, 2527–2535. [Google Scholar] [CrossRef]
- Aalinejad, M.; Pesyan, N.N.; Doustkhah, E. Diaza crown-type macromocycle (kryptofix 22) functionalised carbon nanotube for efficient Ni2+ loading; A unique catalyst for cross-coupling reactions. Mol. Catal. 2020, 494, 111117. [Google Scholar] [CrossRef]
- Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts 2019, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Rostamnia, S.; Doustkhah, E.; Karimi, Z.; Amini, S.; Luque, R. Surfactant-Exfoliated Highly Dispersive Pd-Supported Graphene Oxide Nanocomposite as a Catalyst for Aerobic Aqueous Oxidations of Alcohols. ChemCatChem 2015, 7, 1678–1683. [Google Scholar] [CrossRef]
- Liu, C.; Tan, R.; Yu, N.; Yin, D. Pt–Pd bi-metal nanoparticles captured and stabilized by imine groups in a periodic mesoporous organosilica of SBA-15 for hydrogenation of nitrobenzene. Microporous Mesoporous Mater. 2010, 131, 162–169. [Google Scholar] [CrossRef]
- Sangili, A.; Annalakshmi, M.; Chen, S.-M.; Balasubramanian, P.; Sundrarajan, M. Synthesis of silver nanoparticles decorated on core-shell structured tannic acid-coated iron oxide nanospheres for excellent electrochemical detection and efficient catalytic reduction of hazardous 4-nitrophenol. Compos. B Eng. 2019, 162, 33–42. [Google Scholar] [CrossRef]
- Berillo, D.; Cundy, A. 3D-macroporous chitosan-based scaffolds with in situ formed Pd and Pt nanoparticles for nitrophenol reduction. Carbohydr. Polym. 2018, 192, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Catalyst | Time (min) | Temperature (°C) | TON | Reference |
|---|---|---|---|---|
| TA@Fe3O4-AgNPs | 6 | r.t. * | 25 | [44] |
| PdPt@Chitosan | 120 | 22 | 10 | [45] |
| Pd@MO-urea | 26 | r.t. | 64 | [17] |
| Pd@MO-ISO | 20 | r.t. | 125 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebardasti, A.; Dekamin, M.G.; Doustkhah, E. The Isocyanurate-Carbamate-Bridged Hybrid Mesoporous Organosilica: An Exceptional Anchor for Pd Nanoparticles and a Unique Catalyst for Nitroaromatics Reduction. Catalysts 2021, 11, 621. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050621
Zebardasti A, Dekamin MG, Doustkhah E. The Isocyanurate-Carbamate-Bridged Hybrid Mesoporous Organosilica: An Exceptional Anchor for Pd Nanoparticles and a Unique Catalyst for Nitroaromatics Reduction. Catalysts. 2021; 11(5):621. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050621
Chicago/Turabian StyleZebardasti, Ali, Mohammad G. Dekamin, and Esmail Doustkhah. 2021. "The Isocyanurate-Carbamate-Bridged Hybrid Mesoporous Organosilica: An Exceptional Anchor for Pd Nanoparticles and a Unique Catalyst for Nitroaromatics Reduction" Catalysts 11, no. 5: 621. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050621