Atmospheric Pressure Tornado Plasma Jet of Polydopamine Coating on Graphite Felt for Improving Electrochemical Performance in Vanadium Redox Flow Batteries
Abstract
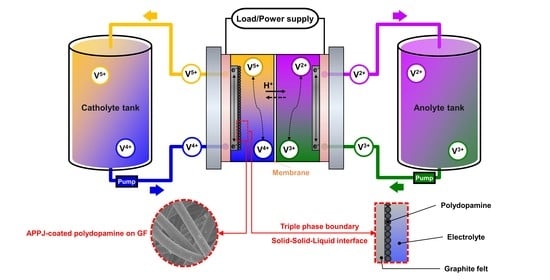
:1. Introduction
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noh, C.; Moon, S.; Chung, Y.; Kwon, Y. Chelating functional group attached to carbon nanotubes prepared for performance enhancement of vanadium redox flow battery. J. Mater. Chem. A 2017, 5, 21334–21342. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, N.H.; Kim, H. Analysis of concentration polarization using UV-visible spectrophotometry in a vanadium redox flow battery. J. Electrochem. Soc. 2014, 161, A1291. [Google Scholar] [CrossRef]
- Chang, T.-C.; Zhang, J.-P.; Fuh, Y.-K. Electrical, mechanical and morphological properties of compressed carbon felt electrodes in vanadium redox flow battery. J. Power Sources 2014, 245, 66–75. [Google Scholar] [CrossRef]
- Ejigu, A.; Edwards, M.; Walsh, D.A. Synergistic Catalyst–Support Interactions in a Graphene–Mn3O4 Electrocatalyst for Vanadium Redox Flow Batteries. ACS Catal. 2015, 5, 7122–7130. [Google Scholar] [CrossRef] [Green Version]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.-Y.; Yang, J.H.; Kim, H.-T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Hammer, E.-M.; Berger, B.; Komsiyska, L. Improvement of the performance of graphite felt electrodes for vanadium-redox-flow-batteries by plasma treatment. Int. J. Renew. Energy Dev. 2014, 3, 7. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent advancements in all-vanadium redox flow batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.; Zhao, G.; An, L.; Zeng, L. A high-performance carbon nanoparticle-decorated graphite felt electrode for vanadium redox flow batteries. Appl. Energy 2016, 176, 74–79. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.; Sun, F.; Zhao, L.; Xing, L. Highly hydroxylated carbon fibres as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.-C.; Wondimu, T.H.; Huang, H.-C.; Wang, C.-H. Carbon and metal-based catalysts for vanadium redox flow batteries: A perspective and review of recent progress. Sustain. Energy Fuels 2021, 5, 1668–1707. [Google Scholar] [CrossRef]
- Cloke, P.L.; Kelly, W.C. Solubility of gold under inorganic supergene conditions. Econ. Geol. 1964, 59, 259–270. [Google Scholar] [CrossRef]
- Lee, W.; Jo, C.; Youk, S.; Shin, H.Y.; Lee, J.; Chung, Y.; Kwon, Y. Mesoporous tungsten oxynitride as electrocatalyst for promoting redox reactions of vanadium redox couple and performance of vanadium redox flow battery. Appl. Surf. Sci. 2018, 429, 187–195. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kim, H. Graphite felt coated with dopamine-derived nitrogen-doped carbon as a positive electrode for a vanadium redox flow battery. J. Electrochem. Soc. 2015, 162, A1675. [Google Scholar] [CrossRef]
- Wei, G.; Jing, M.; Fan, X.; Liu, J.; Yan, C. A new electrocatalyst and its application method for vanadium redox flow battery. J. Power Sources 2015, 287, 81–86. [Google Scholar] [CrossRef]
- Youn, C.; Song, S.A.; Kim, K.; Woo, J.Y.; Chang, Y.-W.; Lim, S.N. Effect of nitrogen functionalization of graphite felt electrode by ultrasonication on the electrochemical performance of vanadium redox flow battery. Mater. Chem. Phys. 2019, 237, 121873. [Google Scholar] [CrossRef]
- Li, Q.; Bai, A.; Zhang, T.; Li, S.; Sun, H. Dopamine-derived nitrogen-doped carboxyl multiwalled carbon nanotube-modified graphite felt with improved electrochemical activity for vanadium redox flow batteries. R. Soc. Open Sci. 2020, 7, 200402. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.L.; Li, S.F.Y. Synergistic effect of the bifunctional polydopamine–Mn3O4 composite electrocatalyst for vanadium redox flow batteries. J. Mater. Chem. A 2017, 5, 15154–15166. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Kencana, S.D.; Su, Y.-M.; Huang, H.-T. Tailoring the O2 reduction activity on hydrangea-like La0.5Sr0.5MnO3 cathode film fabricated via atmospheric pressure plasma jet process. Ceram. Int. 2018, 44, 7349–7356. [Google Scholar] [CrossRef]
- Wang, F.-M.; Kuo, Y.-L.; Huang, L.-S.; Ramar, A.; Su, C.-H. Fabrication of in operando, self-growing, core-shell solid electrolyte interphase on LiFePO4 electrodes for preventing undesirable high-temperature effects in Li-ion batteries. Electrochim. Acta 2018, 268, 260–267. [Google Scholar] [CrossRef]
- Grill, A. Cold Plasma in Materials Fabrication: From Fundamentals to Applications; The Institute of Electrical and Electronics Engineers, Inc.: New York, NY, USA, 1994; pp. 46–85. [Google Scholar]
- Harry, J.E. Elastic and inelastic collision processes in weakly ionized gases. In Introduction to Plasma Technology; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2010; pp. 15–27. [Google Scholar]
- Nist Atomic Spectra Database. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 12 May 2014).
- Jiang, H.; Shyy, W.; Wu, M.; Zhang, R.; Zhao, T. A bi-porous graphite felt electrode with enhanced surface area and catalytic activity for vanadium redox flow batteries. Appl. Energy 2019, 233, 105–113. [Google Scholar] [CrossRef]
- Wu, L.; Shen, Y.; Yu, L.; Xi, J.; Qiu, X. Boosting vanadium flow battery performance by nitrogen-doped carbon nanospheres electrocatalyst. Nano Energy 2016, 28, 19–28. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, J.-Y.; Kabtamu, D.M.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wei, H.-J.; Wang, C.-H. High efficiency of CO2-activated graphite felt as electrode for vanadium redox flow battery application. J. Power Sources 2017, 364, 1–8. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Lee, W.-J.; Wu, Y.-T.; Liao, Y.-W.; Liu, Y.-T. Graphite felt modified by atomic layer deposition with TiO2 nanocoating exhibits super-hydrophilicity, low charge-transform resistance, and high electrochemical activity. Nanomaterials 2020, 10, 1710. [Google Scholar] [CrossRef]
- Li, B.; Gu, M.; Nie, Z.; Wei, X.; Wang, C.; Sprenkle, V.; Wang, W. Nanorod niobium oxide as powerful catalysts for an all vanadium redox flow battery. Nano Lett. 2014, 14, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Al-Fetlawi, H.; Walsh, F. Dynamic modelling of hydrogen evolution effects in the all-vanadium redox flow battery. Electrochim. Acta 2010, 55, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Etesami, M.; Abouzari-Lotf, E.; Ripin, A.; Nasef, M.M.; Ting, T.M.; Saharkhiz, A.; Ahmad, A. Phosphonated graphene oxide with high electrocatalytic performance for vanadium redox flow battery. Int. J. Hydrog. Energy 2018, 43, 189–197. [Google Scholar] [CrossRef]
- Karikalan, N.; Elavarasan, M.; Yang, T.C. Effect of cavitation erosion in the sonochemical exfoliation of activated graphite for electrocatalysis of acebutolol. Ultrason. Sonochem. 2019, 56, 297–304. [Google Scholar] [CrossRef]
- Jiang, H.; Shyy, W.; Wu, M.; Wei, L.; Zhao, T. Highly active, bi-functional and metal-free B4C-nanoparticle-modified graphite felt electrodes for vanadium redox flow batteries. J. Power Sources 2017, 365, 34–42. [Google Scholar] [CrossRef]
- Ciubuc, J.D.; Qiu, C.; Bennet, K.E.; Alonzo, M.; Durrer, W.; Manciu, F.S. Raman computational and experimental studies of dopamine molecules on silver nanocolloids. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 153–158. [Google Scholar]
- He, B.; Li, G.; Chen, L.; Chen, Z.; Jing, M.; Zhou, M.; Zhou, N.; Zeng, J.; Hou, Z. A facile N doping strategy to prepare mass-produced pyrrolic N-enriched carbon fibers with enhanced lithium storage properties. Electrochim. Acta 2018, 278, 106–113. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Chen, J.-Y.; Chang, Y.-C.; Wang, C.-H. Electrocatalytic activity of Nb-doped hexagonal WO3 nanowire-modified graphite felt as a positive electrode for vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 11472–11480. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Duan, W.; Wang, W.; Lin, Y.; Wang, Y.; Liu, J. Nanostructured electrocatalysts for PEM fuel cells and redox flow batteries: A selected review. ACS Catal. 2015, 5, 7288–7298. [Google Scholar] [CrossRef]
- Ghimire, P.C.; Schweiss, R.; Scherer, G.G.; Wai, N.; Lim, T.M.; Bhattarai, A.; Nguyen, T.D.; Yan, Q. Titanium carbide-decorated graphite felt as high performance negative electrode in vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 6625–6632. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, S.-W.; Yim, T.; Kim, J.-G.; Choi, J.W.; Kim, J.H.; Park, M.-S.; Kim, Y.-J. A new strategy for integrating abundant oxygen functional groups into carbon felt electrode for vanadium redox flow batteries. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.-C.; Chen, G.-C.; Chen, H.-Y.; Lin, G.-Y.; Liu, T.-R.; Wondimu, T.H.; Wang, K.-C.; Wang, C.-H. Synergistic effects of a TiNb2O7–reduced graphene oxide nanocomposite electrocatalyst for high-performance all-vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 13908–13917. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Myung, S.-W.; Lee, C.-S.; Choi, H.-S. Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 2006, 295, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, Y.; Zhang, C.; Yan, P.; Shao, T. Comparison between helium and argon plasma jets on improving the hydrophilic property of PMMA surface. Appl. Surf. Sci. 2016, 367, 401–406. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Li, X.; Liu, Y.; Shi, Z.; Wang, Y. Synthesis and mechanical properties of dopamine modified titanium dioxide/waterborne polyurethane composites. Polym. Compos. 2019, 40, 328–336. [Google Scholar] [CrossRef]
- Hofman, A.H.; van Hees, I.A.; Yang, J.; Kamperman, M. Bioinspired underwater adhesives by using the supramolecular toolbox. Adv. Biomater. 2018, 30, 1704640. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Min, J.; Lin, J.; Wan, H. Effect of atmospheric pressure plasma treatment on adhesive bonding of carbon fiber reinforced polymer. Polymers 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Wang, N.; Wu, Z.; Li, L. Probing active sites on metal-free, nitrogen-doped carbons for oxygen electroreduction: A review. Catalysts 2018, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Jawaid, M.; Khan, A.A.P.; Asiri, A.M. Electrically Conductive Polymers and Polymer Composites: From Synthesis to Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Tseng, T.-M.; Huang, R.-H.; Huang, C.-Y.; Hsueh, K.-L.; Shieu, F.-S. A Kinetic Study of the Platinum/Carbon Anode Catalyst for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2013, 160, A690. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-H.; Sun, C.-H.; Tseng, T.-m.; Chao, W.-K.; Hsueh, K.-L.; Shieu, F.-S. Investigation of active electrodes modified with platinum/multiwalled carbon nanotube for vanadium redox flow battery. J. Electrochem. Soc. 2012, 159, A1579. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Kwon, Y. Performance enhancement in vanadium redox flow battery using platinum-based electrocatalyst synthesized by polyol process. Electrochim. Acta 2013, 114, 439–447. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim. Acta 2007, 52, 6755–6762. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, J.-H.; Hwang, U.; Lee, N.J.; Jeong, G.; Kim, Y.-J. Novel catalytic effects of Mn3O4 for all vanadium redox flow batteries. Chem. Commun. 2012, 48, 5455–5457. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.T.; Jo, C.; Lee, J.; Kwon, Y. MoO2 nanocrystals interconnected on mesocellular carbon foam as a powerful catalyst for vanadium redox flow battery. RSC Adv. 2016, 6, 17574–17582. [Google Scholar] [CrossRef]











| Current density (mA/cm2) | CE (%) | VE (%) | EE (%) |
|---|---|---|---|
| 40 | 85.24 | 93.83 | 79.86 |
| 60 | 91.90 | 86.15 | 79.16 |
| 80 | 93.81 | 81.39 | 76.31 |
| 100 | 95.11 | 77.21 | 73.42 |
| 120 | 96.02 | 69.84 | 67.02 |
| Catalyst/Electrode | Current Density (mA/cm2) | Efficiency | Ref | ||
|---|---|---|---|---|---|
| CE (%) | VE (%) | EE (%) | |||
| Pt-C/CF | 10 | 80.6 | 89.7 | 72.3 | [49] |
| Pt/MWNTs | 20 | 83.88 | 27.55 | 23.11 | [50] |
| Pt-C/sprayed GF | 40 | 87 | 84 | 71 | [51] |
| Ir/GF | 40 | 82 | 74.6 | 61.1 | [52] |
| Mn3O4/CF | 40 | 83.5 | 91 | 76 | [53] |
| MoO2/MSU-FC | 80 | 91 | 79.7 | 72.5 | [54] |
| APPJ polydopamine coating/GF | 40 | 85.24 | 93.83 | 79.86 | This work |
| 80 | 93.81 | 81.39 | 76.31 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Kuo, Y.-L.; Wang, Y.-M.; Hsu, W.-M.; Chien, T.-H.; Lin, C.-F.; Kuo, C.-H.; Okino, A.; Chiang, T.-C. Atmospheric Pressure Tornado Plasma Jet of Polydopamine Coating on Graphite Felt for Improving Electrochemical Performance in Vanadium Redox Flow Batteries. Catalysts 2021, 11, 627. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050627
Chen S-Y, Kuo Y-L, Wang Y-M, Hsu W-M, Chien T-H, Lin C-F, Kuo C-H, Okino A, Chiang T-C. Atmospheric Pressure Tornado Plasma Jet of Polydopamine Coating on Graphite Felt for Improving Electrochemical Performance in Vanadium Redox Flow Batteries. Catalysts. 2021; 11(5):627. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050627
Chicago/Turabian StyleChen, Song-Yu, Yu-Lin Kuo, Yao-Ming Wang, Wei-Mau Hsu, Tzu-Hsuan Chien, Chiu-Feng Lin, Cheng-Hsien Kuo, Akitoshi Okino, and Tai-Chin Chiang. 2021. "Atmospheric Pressure Tornado Plasma Jet of Polydopamine Coating on Graphite Felt for Improving Electrochemical Performance in Vanadium Redox Flow Batteries" Catalysts 11, no. 5: 627. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050627