Kinetic Study of 17α-Estradiol Activity in Comparison with 17β-Estradiol and 17α-Ethynylestradiol
Abstract
:1. Introduction
2. Results
2.1. Monitoring the αE2 Concentration during Time-Depedent Capacitation Using the HPLC-MS/MS Method
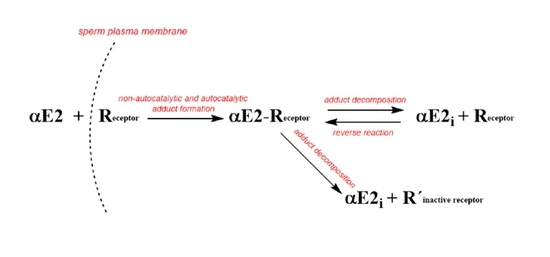
2.2. Kinetic Analysis of the HPLC-MS/MS Data
- (i)
- The initial branch of the hypothetical curve that would be obtained by fitting the experimental points is the opposite to that of the autocatalytic curve.
- (ii)
- The slope of the tangent to this hypothetical curve decreases in its initial region to zero with time.
- (iii)
- The determined value of n is too low for a dose hormone concentration of 200 μg/L.
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Animals
4.2. Instrumentation and Chromatographic Conditions
4.3. Sample Preparation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López de Alda, M.J.; Barceló, D. Determination of steroid sex hormones and related synthetic compounds considered as endocrine disruptors in water by liquid chromatography-diode array detection-mass spectrometry. J. Chromatogr. A 2000, 892, 391–406. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Della Seta, D.; Farabollini, F.; Dessí-Fulgheri, F.; Fusani, L. Environmental-like exposure to low levels of estrogen affects sexual behavior and physiology of female rats. Endocrinology 2008, 149, 5592–5598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef] [Green Version]
- Aquila, S.; De Amicis, F. Steroid receptors and their ligands: Effects on male gamete functions. Exp. Cell Res. 2014, 328, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, P.; Zatecka, E.; Dvorakova-Hortova, K. Of oestrogens and sperm: A review of the roles of oestrogens and oestrogen receptors in male reproduction. Int. J. Mol. Sci. 2017, 18, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Majumdar, C.; Roy, P. Effects of endocrine disrupting chemicals from leather industry effluents on male reproductive system. J. Steroid Biochem. Mol. Biol. 2008, 111, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Toppari, J.; Keiding, N.; Skakkebaek, N.E. Do environmental estrogens contribute to the decline in male reproductive health? Clin. Chem. 1995, 41, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, A.D.; Schug, K.A. A review of separation methods for the determination of estrogens and plastics-derived estrogen mimics from aqueous systems. Anal. Chim. Acta 2011, 696, 6–26. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Harrison, D.E. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.P.; McGuire, W.L. 17 alpha-Estradiol is a biologically active estrogen in human breast cancer cells in tissue culture. Endocrinology 1980, 107, 884–891. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Lee, W.-S. Male Alopecia: Guide to Successful Management; Springer Science & Business Media: New York, NY, USA, 2014; p. 93. [Google Scholar]
- Stout, M.B.; Steyn, F.J.; Jurczak, M.J.; Camporez, J.G.; Zhu, Y.; Hawse, J.R.; Jurk, D.; Palmer, A.K.; Xu, M.; Pirtskhalava, T.; et al. 17α-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.; Liu, R.; Yang, S.H.; Cai, Z.Y.; Covey, D.F.; Simpkins, J.W. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005, 1038, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Toran-Allerand, C.D.; Guan, X.; MacLusky, N.J.; Horvath, T.L.; Diano, S.; Singh, M.; Tinnikov, A.A. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002, 22, 8391–8401. [Google Scholar] [CrossRef] [PubMed]
- Perusquía, M.; Navarrete, E. Evidence that 17alpha-estradiol is biologically active in the uterine tissue: Antiuterotonic and antiuterotrophic action. Reprod. Biol. Endocrinol. 2005, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Sievernich, A.; Wildt, L.; Lichtenberg-Frate, H. In vitro bioactivity of 17α-estradiol. J. Steroid Biochem. Mol. Biol. 2004, 92, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.; Leander, D.; Pifer, K.; Bower, B.; Herrera, J.J.; Day, S.M.; Fiehn, O.; Brooks, S.V.; Miller, R.A. 17-α estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell 2019, 18, 2. [Google Scholar] [CrossRef]
- Moos, W.H.; Dykens, J.A.; Nohynek, D.; Rubinchik, E.; Howell, N. Review of the effects of 17α-estradiol in humans: A less feminizing estrogen with neuroprotective potential. Drug Dev. Res. 2009, 70, 1–21. [Google Scholar] [CrossRef]
- Schriefers, H.; Wright, M.C.; Rozman, T.; Hevert, F. Inhibition of testosterone metabolism by 17-alpha-estradiol in rat liver slices. Arzneimittelforschung 1991, 41, 1186–1189. [Google Scholar]
- Mann, S.N.; Pitel, K.S.; Nelson-Holte, M.H.; Iwaniec, U.T.; Turner, R.T.; Sathiaseelan, R.; Kirkland, J.L.; Schneider, A.; Morris, K.T.; Malayannan, S.; et al. 17α-Estradiol prevents ovariectomy-mediated obesity and bone loss. Exp. Gerontol. 2020, 142. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Reifsnyder, P.; Kumar, N.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Lopez-Cruzan, M.; Macchiarini, F.; Nelson, J.F. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 2021. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.; Bower, B.; Garcia, G.G.; Miller, R.A. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: Gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 2017, 16, 1256–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isola, J.V.V.; Zanini, B.M.; Sidhom, S.; Kopchick, J.J.; Bartke, A.; Masternak, M.M.; Stout, M.B.; Schneider, A. 17α-Estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp. Gerontol. 2020, 129. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.M.; Hadad, N.; Holte, M.N.; Rothman, A.R.; Sathiaseelan, R.; Mondal, S.A.; Agbaga, M.-P.; Unnikrishnan, A.; Subramaniam, M.; Hawse, J.; et al. Health benefits attributed to 17a-estradiol, a lifespan-extending compound, are mediated through estrogen receptor a. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, L.; Moore, G.D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Yanagimachi, R. The Physiology of Reprodution; Knobil, E.J.D.N., Ed.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Palumbo, M.C.; Farina, L.; Paci, P. Kinetics effects and modeling of mRNA turnover. WIREs RNA 2015, 6, 327–336. [Google Scholar] [CrossRef]
- Cao, D.; Parker, R. Computation modeling of eukaryotic mRNA turnover. RNA 2001, 7, 1192–1212. [Google Scholar] [CrossRef] [Green Version]
- Bosakova, T.; Tockstein, A.; Sebkova, S.O.; Adamusova, H.; Albrechtova, J.; Albrecht, T.; Bosakova, Z.; Dvorakova-Hortova, K. New insight into sperm capacitation: A novel mechanism of 17β-estradiol signalling. Int. J. Mol. Sci. 2018, 19, 4011. [Google Scholar] [CrossRef] [Green Version]
- Bosakova, T.; Tockstein, A.; Sebkova, N.; Cabala, R.; Komrskova, K. Kinetic model of the action of 17α-ethynylestradiol on the capacitation of mouse sperm, monitored by HPLC-MS/MS. Catalysts 2020, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Notas, G.; Pelekanou, V.; Troullinaki, M.; Andrianaki, M.; Azariadis, K.; Kampouri, E.; Lavrentaki, K.; Castanas, E. Early membrane initiated transcriptional effects of estrogens in breast cancer cells: First pharmacological evidence for a novel membrane estrogen receptor element (ERX). Steroids 2012, 77, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Ded, L.; Sebkova, N.; Cerna, M.; Elyeinova, F.; Dostalova, P.; Peknicova, J.; Dvorakova-Hortova, K. In vivo exposure to 17beta-estradiol triggers premature sperm capacitation in cauda epidimis. Reproduction 2013, 145, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: Genetic and environmental aspects. Hum. Reprod. Update 2006, 12, 303–323. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, A.; Ganz, F.; Pengo, M.; Selice, R.; Frigo, A.C.; Foresta, C. Association of testicular germ cell tumor with polymorphisms in estrogen receptor and steroid metabolism genes. Endocr. Relat. Cancer 2010, 17, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Lermontova, N.N.; P’chev, V.K.; Beznosko, B.K.; Van’kin, G.I.; Ivanova, T.A.; Koroleva, I.V.; Lukoyanova, E.A.; Mukhina, T.V.; Serkova, T.P.; Bachurin, S.O. Effects of 17 beta-estradiol and its isomer 17 alpha-estradiol on learning in rats with chronic cholinergic deficiency in the brain. Bull. Exp. Biol. Med. 2000, 129, 442–444. [Google Scholar] [CrossRef] [PubMed]
- McClean, J.; Nuñez, J.L. 17alpha-Estradiol is neuroprotective in male and female rats in a model of early brain injury. Exp. Neurol. 2008, 210, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Kozlík, P.; Bosáková, Z.; Tesařová, E.; Coufal, P.; Čabala, R. Development of a solid-phase extraction with capillary liquid chromatography tandem mass spectrometry for analysis of estrogens in environmental water samples. J. Chromatogr. A 2011, 1218, 2127–2132. [Google Scholar] [CrossRef]




| Time (min) | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
|---|---|---|---|---|---|---|---|
| Bt | 1.000 ± 0.002 | 0.985 ± 0.002 | 0.981 ± 0.003 | 0.979 ± 0.008 | 0.899 ± 0.010 | 0.772 ± 0.012 | 0.863 ± 0.013 |
| K1 | K2 | K3 | K4 | K5 | n |
|---|---|---|---|---|---|
| 0.01 | 4.0 | 5.0 | 0 | 0 | 0.01 |
| K1 | K2 | K3 | K4 | K5 | n |
|---|---|---|---|---|---|
| 7 | 10 | 49 | 12 | 20 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosakova, T.; Tockstein, A.; Bosakova, Z.; Komrskova, K. Kinetic Study of 17α-Estradiol Activity in Comparison with 17β-Estradiol and 17α-Ethynylestradiol. Catalysts 2021, 11, 634. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050634
Bosakova T, Tockstein A, Bosakova Z, Komrskova K. Kinetic Study of 17α-Estradiol Activity in Comparison with 17β-Estradiol and 17α-Ethynylestradiol. Catalysts. 2021; 11(5):634. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050634
Chicago/Turabian StyleBosakova, Tereza, Antonin Tockstein, Zuzana Bosakova, and Katerina Komrskova. 2021. "Kinetic Study of 17α-Estradiol Activity in Comparison with 17β-Estradiol and 17α-Ethynylestradiol" Catalysts 11, no. 5: 634. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050634