Influence of Co-Precipitation Agent on the Structure, Texture and Catalytic Activity of Au-CeO2 Catalysts in Low-Temperature Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Results
2.1. Characterization of Materials
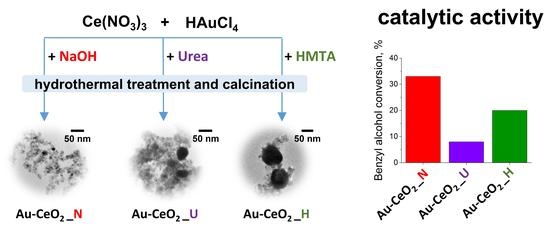
2.2. Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Catalysts
4.3. Characterization of Materials
4.4. Catalytic Activity Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy Between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Effect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Cui, Y.; Dai, W.-L. Support morphology and crystal plane effect of Cu/CeO2 nanomaterial on the physicochemical and catalytic properties for carbonate hydrogenation. Catal. Sci. Technol. 2016, 6, 7752–7762. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Xu, W.; Li, X.; Wang, W.; Zhang, L.; Li, Y.; Peng, Z.; Yang, F.; Liu, Z. Nature of Active Sites on Cu–CeO2 Catalysts Activated by High-Temperature Thermal Aging. ACS Catal. 2020, 10, 12385–12392. [Google Scholar] [CrossRef]
- Grabchenko, M.; Mikheeva, N.; Mamontov, G.; Salaev, M.; Liotta, L.; Vodyankina, O. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Negi, K.; Umar, A.; Chauhan, M.S.; Akhtar, M.S. Ag/CeO2 nanostructured materials for enhanced photocatalytic and antibacterial applications. Ceram. Int. 2019, 45, 20509–20517. [Google Scholar] [CrossRef]
- Zhu, X.; He, H.; Li, Y.; Wu, H.; Fu, M.; Ye, D.; Wu, J.; Huang, H.; Hu, Y.; Niu, X. CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability. Nanomaterials 2020, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Wang, Q.; Zhou, X.; Li, J.; Song, S.; Zhang, H. CeO2 supported low-loading Au as an enhanced catalyst for low temperature oxidation of carbon monoxide. CrystEngComm 2019, 21, 7108–7113. [Google Scholar] [CrossRef]
- Xiang, Y.; He, J.; Sun, N.; Fan, Y.; Yang, L.; Fang, C.; Kuai, L. Hollow mesoporous CeO2 microspheres for efficient loading of Au single-atoms to catalyze the water-gas shift reaction. Microporous Mesoporous Mater. 2020, 308, 110507. [Google Scholar] [CrossRef]
- Carltonbird, M.; Eaimsumang, S.; Pongstabodee, S.; Boonyuen, S.; Smith, S.M.; Luengnaruemitchai, A. Effect of the exposed ceria morphology on the catalytic activity of gold/ceria catalysts for the preferential oxidation of carbon monoxide. Chem. Eng. J. 2018, 344, 545–555. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshri, K.S.; Spezzati, G.; Ruidas, S.; Hensen, E.J.M.; Chowdhury, B. Role of bismuth on aerobic benzyl alcohol oxidation over ceria polymorph-supported gold nanoparticles. Catal. Commun. 2020, 140, 106004. [Google Scholar] [CrossRef]
- Kepeniene, V.; Stagniunaite, R.; Balčiunaite, A.; Tamašauskaite-Tamašiunaite, L.; Norkus, E. Microwave-assisted synthesis of a AuCeO2/C catalyst and its application for the oxidation of alcohols in an alkaline medium. New J. Chem. 2020, 44, 18308–18318. [Google Scholar] [CrossRef]
- Dong, Y.; Luo, J.; Li, S.; Liang, C. CeO2 decorated Au/CNT catalyst with constructed Au-CeO2 interfaces for benzyl alcohol oxidation. Catal. Commun. 2020, 133, 1–5. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Y.; Jiang, G.; Hou, X.; Li, S.; Zhang, Z. Understanding of Au-CeO2 interface and its role in catalytic oxidation of formaldehyde. Appl. Catal. B Environ. 2020, 260, 118138. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhou, J.; Liu, Y.; Wei, Z.; Zhang, H. Layered double hydroxides supported atomically precise Aun nanoclusters for air oxidation of benzyl alcohol: Effects of size and active site structure. J. Catal. 2020, 389, 409–420. [Google Scholar] [CrossRef]
- Engel, J.; Schwartz, E.; Catlow, C.R.A.; Roldan, A. The influence of oxygen vacancy and Ce3+ ion positions on the properties of small gold clusters supported on CeO2-x(111). J. Mater. Chem. A 2020, 8, 15695–15705. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharjee, G.; Satpati, B.; Kumar, M.; Deka, S.; Ghosalya, M.K.; Gopinath, C.; Bala, T. Deposition of Au nanoparticles inside porous CeO2 nanocubes using Langmuir-Blodgett technique. New J. Chem. 2018, 42, 1379–1386. [Google Scholar] [CrossRef]
- Piella, J.; Gónzalez-Febles, A.; Patarroyo, J.; Arbiol, J.; Bastús, N.G.; Puntes, V. Seeded-Growth Aqueous Synthesis of Colloidal-Stable Citrate-Stabilized Au/CeO2 Hybrid Nanocrystals: Heterodimers, Core@Shell, and Clover- And Star-Like Structures. Chem. Mater. 2019, 31, 7922–7932. [Google Scholar] [CrossRef] [Green Version]
- Sudarsanam, P.; Mallesham, B.; Durgasri, D.N.; Reddy, B.M. Physicochemical and catalytic properties of nanosized Au/CeO2 catalysts for eco-friendly oxidation of benzyl alcohol. J. Ind. Eng. Chem. 2014, 20, 3115–3121. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, F.X.; Gao, W.; Dong, Q.C.; Qu, Y.Q. Uniform small metal nanoparticles anchored on CeO2 nanorods driven by electroless chemical deposition. Rare Met. 2020, 39, 806–814. [Google Scholar] [CrossRef]
- Baatz, C.; Thielecke, N.; Prüße, U. Influence of the preparation conditions on the properties of gold catalysts for the oxidation of glucose. Appl. Catal. B Environ. 2007, 70, 653–660. [Google Scholar] [CrossRef]
- Radnik, J.; Wilde, L.; Schneider, M.; Pohl, M.M.; Herein, D. Influence of the precipitation agent in the deposition-precipitation on the formation and properties of Au nanoparticles supported on Al2O3. J. Phys. Chem. B 2006, 110, 23688–23693. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Giorgio, S.; Shin, C.H.; Henry, C.R.; Louis, C. Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J. Catal. 2004, 222, 357–367. [Google Scholar] [CrossRef]
- Chen, B.B.; Shi, C.; Crocker, M.; Wang, Y.; Zhu, A.M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts. Appl. Catal. B Environ. 2013, 132–133, 245–255. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; He, Q.; Zeng, S.; Sun, H.; Liu, Z. Controlled preparation and visible light photocatalytic activities of corn cob-like Au-ZnO nanorods. J. Mater. Sci. 2017, 52, 3478–3489. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Kolodziejczak-Radzimska, A.; Jesionowski, T.; Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Md Saad, S.K.; Ali Umar, A.; Ali Umar, M.I.; Tomitori, M.; Rahman, M.Y.A.; Mat Salleh, M.; Oyama, M. Two-Dimensional, Hierarchical Ag-Doped TiO2 Nanocatalysts: Effect of the Metal Oxidation State on the Photocatalytic Properties. ACS Omega 2018, 3, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Tajizadegan, H.; Heidary, A.; Torabi, O.; Golabgir, M.H.; Jamshidi, A. Synthesis and Characterization of ZnCr2O4 Nanospinel Prepared via Homogeneous Precipitation Using Urea Hydrolysis. Int. J. Appl. Ceram. Technol. 2016, 13, 289–294. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, M.; Li, D.; Tian, Y.; Morioka, H.; Honda, M.; Sano, T.; Takehira, K. Water-gas shift reaction over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation. Appl. Catal. A Gen. 2006, 303, 62–71. [Google Scholar] [CrossRef]
- Rosado, T.F.; Teixeira, M.P.; Moraes, L.C.; da Silva, L.A.; Pontes-Silva, A.V.; Taylor, J.G.; de Freitas, I.C.; de Oliveira, D.C.; Gardener, J.; Solórzano, G.; et al. Synergistic Effect between CeO2 Nanowires and Gold NPs over the Activity and Selectivity in the Oxidation of Thioanisole. Appl. Catal. A Gen. 2021, 118010. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhu, T.; Li, N.; Xu, C.W. Glycerol Electrooxidation on Au Supported on Carbon Spheres by Stober Method in Alkaline Medium. Int. J. Electrochem. Sci. 2013, 8, 9508–9517. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yao, Z.; Liu, B.; Dong, L. Correlation of structural characteristics with catalytic performance of CuO/CexZr1-xO2 catalysts for NO reduction by CO. J. Catal. 2010, 275, 45–60. [Google Scholar] [CrossRef]
- Reddy, B.M.; Bharali, P.; Saikia, P.; Park, S.E.; Van Den Berg, M.W.E.; Muhler, M.; Grünert, W. Structural characterization and catalytic activity of nanosized CexM1-xO2 (M = Zr and Hf) mixed oxides. J. Phys. Chem. C 2008, 112, 11729–11737. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A comparative study of Cu, Ag and Au doped CeO2 in the total oxidation of volatile organic compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
- Corro, G.; Cebada, S.; Pal, U.; Fierro, J.L.G. Au0–Au3+ bifunctional site mediated enhanced catalytic activity of Au/ZnO composite in diesel particulate matter oxidation. J. Catal. 2017, 347, 148–156. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Prati, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45, 4953–4994. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Blouin, M.; Pillonnet, A.; Guay, D. Structure and valence properties of ceria films synthesized by laser ablation under reducing atmosphere. Mater. Res. Express 2014, 1, 015704. [Google Scholar] [CrossRef]
- Ismail, A.A.; Harraz, F.A.; Faisal, M.; El-Toni, A.M.; Al-Hajry, A.; Al-Assiri, M.S. A sensitive and selective amperometric hydrazine sensor based on mesoporous Au/ZnO nanocomposites. Mater. Des. 2016, 109, 530–538. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]










| Catalyst | Real Gold Loading 1 (wt.%) | BET Surface Area 2 (m2/g) | Average Pore Size 3 (nm) | Average Gold Particle Size 4 (nm) |
|---|---|---|---|---|
| CeO2_U | - | 46 | 6.9 | - |
| Au-CeO2_U | 4.3 | 35 | 6.5 | 61.8 |
| CeO2_H | - | 11 | 17.1 | - |
| Au-CeO2_H | 4.7 | 28 | 12.0 | 51.5 |
| CeO2_N | - | 49 | 8.1 | - |
| Au-CeO2_N | 4.5 | 45 | 7.4 | 12.5 |
| Catalyst | Reaction Time [Min] | Conversion [%] | Selectivity [%] | |
|---|---|---|---|---|
| Benzaldehyde | Benzoic Acid | |||
| CeO2_U | 40 | - | - | - |
| Au-CeO2_U | 40 | 17 | 56 | 44 |
| CeO2_H | 40 | - | - | - |
| Au-CeO2_H | 20 | 20 | 52 | 48 |
| 40 | 38 | 22 | 78 | |
| CeO2_N | 40 | << 1 | - | traces |
| Au-CeO2_N | 20 | 33 | 14 | 86 |
| 40 | 47 | 9 | 91 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolski, L.; Nowaczyk, G.; Jurga, S.; Ziolek, M. Influence of Co-Precipitation Agent on the Structure, Texture and Catalytic Activity of Au-CeO2 Catalysts in Low-Temperature Oxidation of Benzyl Alcohol. Catalysts 2021, 11, 641. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050641
Wolski L, Nowaczyk G, Jurga S, Ziolek M. Influence of Co-Precipitation Agent on the Structure, Texture and Catalytic Activity of Au-CeO2 Catalysts in Low-Temperature Oxidation of Benzyl Alcohol. Catalysts. 2021; 11(5):641. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050641
Chicago/Turabian StyleWolski, Lukasz, Grzegorz Nowaczyk, Stefan Jurga, and Maria Ziolek. 2021. "Influence of Co-Precipitation Agent on the Structure, Texture and Catalytic Activity of Au-CeO2 Catalysts in Low-Temperature Oxidation of Benzyl Alcohol" Catalysts 11, no. 5: 641. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050641