Reagent grade solvents and all materials for synthesis, purification, characterization, and catalysis were purchased from commercial sources and used as received unless otherwise stated. Iridium(III) chloride hydrate was purchased from Pressure Chemical (Pittsburgh, PA, USA). 1,3-Diisopropylimidazolium chloride, l-phenylglycine, acetophenone, 4’-chloroacetophenone, and 4’-methylacetophenone were purchased from Sigma Aldrich (St. Louis, MO, USA). l-Alanine, l-pipecolic acid, d-proline, l-proline, l-valine, cyclohexyl phenyl ketone, 4’-fluoroacetophenone, isobutyrophenone, propiophenone, 4’-(trifluoromethyl)acetophenone, and 2,2,2-trimethylacetophenone were purchased from Alfa Aesar (Ward Hill, MA, USA). l-Azetidine-2-carboxylic acid and cis-4-fluoro-l-proline were purchased from Indofine Chemical (Hillsborough, NJ, USA). Glycine was purchased Qiagen Sciences (Germanstown, MD 20874, USA). Deuterated solvents for NMR spectroscopy were obtained from Cambridge Isotope Laboratories (Andover, MA, USA).
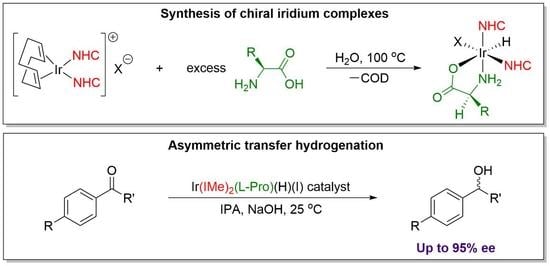
3.2. General Procedure for the Synthesis of Ir(NHC)2(aa)(H)(X) Complexes
A Schlenk flask was charged with the desired iridium COD bis-NHC complex, amino acid, and 15 mL of water. Upon heating the mixture to 100 °C, both reagents fully dissolved, and solution was allowed to react for period of 12 h, over which time the color changed from bright orange to light yellow. The water was removed by rotary evaporation, and the crude product was extracted into dichloromethane. After drying with magnesium sulfate, the mixture was filtered through Celite, and the dichloromethane was removed by rotary evaporation. Following trituration with diethyl ether, the products were isolated on a fritted glass filter as off-white solids, and dried overnight under vacuum.
3.2.1. Synthesis of Ir(IMe)2(Gly)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and glycine (0.0500 g, 0.6666 mmol) were reacted in water to give Ir(IMe)2(Gly)(H)(I) (0.0999 g, 53%). H NMR (400 MHz, DMSO-d6, δ): 7.45 (d, J = 2.0 Hz, 1H, NHC backbone), 7.33 (d, J = 2.0 Hz, 1H, NHC backbone), 7.31 (d, J = 2.0 Hz, 1H, NHC backbone), 7.27 (d, J = 1.9 Hz, 1H, NHC backbone), 5.21 (m, 1H, NH), 4.94 (m, 1H, NH), 4.06 (s, 3H, NHC CH), 3.80 (s, 3H, NHC CH), 3.33–3.25 (m, 1H, αCH), 3.18 (s, 3H, NHC CH), 3.17–3.10 (m, 1H, αCH), 2.89 (s, 3H, NHC CH), −24.33 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 178.8 (C(O)), 149.4 (Ccarbene), 143.5 (Ccarbene), 123.5 (NHC backbone), 123.2 (NHC backbone), 122.8 (NHC backbone), 122.7 (NHC backbone), 47.0 (αC), 38.3 (NHC CH), 38.2 (NHC CH), 36.8 (NHC CH), 35.0 (NHC CH). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 460.1319; Found: 460.1304.
3.2.2. Synthesis of Ir(IMe)2(l-Ala)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-alanine (0.0575 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Ala)(H)(I) (0.1503 g, 78%). Analysis for major isomer (51%): H NMR (400 MHz, DMSO-d6, δ): 7.46 (d, J = 1.9 Hz, 1H, NHC backbone), 7.42 (d, J = 1.9 Hz, 1H, NHC backbone), 7.32 (d, J = 1.9 Hz, 1H, NHC backbone), 7.29 (d, J = 2.0 Hz, 1H, NHC backbone), 5.07 (dd, J = 12.5, 6.1 Hz, 1H, NH), 4.29 (dd, J = 12.7, 9.6 Hz, 1H, NH), 4.11 (s, 3H, NHC CH), 3.76 (s, 3H, NHC CH), 3.19 (s, 3H, NHC CH), 2.98 (s, 3H, NHC CH), 2.55–2.41 (m, 1H, αCH), 1.23 (d, J = 7.0 Hz, 3H, ala CH), −24.00 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 179.9 (C(O)), 149.0 (Ccarbene), 142.1 (Ccarbene), 123.3 (NHC backbone), 123.2 (NHC backbone), 122.8 (NHC backbone), 122.7 (NHC backbone), 53.7 (αC), 38.6 (NHC CH), 38.2 (NHC CH), 36.8 (NHC CH), 35.2 (NHC CH), 20.6 (ala CH). Analysis for minor isomer (49%): H NMR (400 MHz, DMSO-d6, δ): 7.34 (d, J = 2.0 Hz, 1H, NHC backbone), 7.32 (d, J = 2.0 Hz, 1H, NHC backbone), 7.25 (d, J = 1.9 Hz, 1H, NHC backbone), 7.24 (d, J = 1.9 Hz, 1H, NHC backbone), 5.59 (dd, J = 11.6, 5.1 Hz, 1H, NH), 4.52 (t, J = 11.4 Hz, 1H, NH), 4.06 (s, 3H, NHC CH), 3.94 (s, 3H, NHC CH), 3.41 (ddt, J = 13.9, 10.0, 6.9 Hz, 1H, αCH), 3.18 (s, 3H, NHC CH), 2.86 (s, 3H, NHC CH), 1.14 (d, J = 6.9 Hz, 3H, Ala CH), −24.23 (s, 1H, hydride). CNMR (101 MHz, DMSO-d6, δ): 179.0 (C(O)), 149.0 (Ccarbene), 143.6 (Ccarbene), 123.8 (NHC backbone), 123.3 (NHC backbone), 122.9 (NHC backbone), 122.8 (NHC backbone), 53.3 (αC), 38.7 (NHC CH), 38.3 (NHC CH), 36.6 (NHC CH), 34.9 (NHC CH), 20.0 (Ala CH). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 474.1476; Found: 474.1455.
3.2.3. Synthesis of Ir(IMe)2(l-Val)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-valine (0.0756 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Val)(H)(I) (0.1756 g, 87%). Analysis for major isomer (55 %): H NMR (400 MHz, DMSO-d6, δ): 7.44 (d, J = 2.0 Hz, 1H, NHC backbone), 7.36 (d, J = 2.0 Hz, 1H, NHC backbone), 7.28 (d, J = 2.0 Hz, 1H, NHC backbone), 7.26 (d, J = 2.0 Hz, 2H, NHC backbone), 4.78 (dd, J = 12.5, 6.2 Hz, 1H, NH), 4.17 (br d, J = 12.4 Hz, 1H, NH), 4.13 (s, 3H, NHC CH), 3.96 (s, 3H, NHC CH), 3.25 (s, 3H, NHC CH), 3.10 (s, 3H, NHC CH), 2.32 (ddd, J = 11.2, 6.0, 3.1 Hz, 1H, αCH), 2.13 (ddp, J = 9.9, 6.9, 3.5 Hz, 1H, Val CH), 0.93 (d, J = 7.1 Hz, 3H, Val CH), 0.83 (d, J = 6.9 Hz, 3H, Val CH), −23.80 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 178.5 (C(O)), 148.0 (Ccarbene), 141.2 (Ccarbene), 124.0 (NHC backbone), 123.9 (NHC backbone), 123.0 (NHC backbone), 122.9 (NHC backbone), 62.1 (αC), 38.7 (NHC CH), 38.4 (NHC CH), 36.9 (NHC CH), 35.3 (NHC CH), 31.5 (Val CH), 18.4 (Val CH), 16.9 (Val CH). Analysis for minor isomer (45%): H NMR (400 MHz, DMSO-d6, δ): 7.45 (d, J = 2.0 Hz, 1H, NHC backbone), 7.34 (d, J = 2.0 Hz, 1H, NHC backbone), 7.31 (d, J = 2.0 Hz, 1H, NHC backbone), 7.26 (d, J = 1.7 Hz, 2H, NHC backbone), 5.43 (dd, J = 12.0, 6.6 Hz, 1H, NH), 4.09 (s, 3H, NHC CH), 3.89 (s, 3H, NHC CH), 3.23 (s, 3H, NHC CH), 3.16 (ddd, J = 9.0, 6.7, 4.5 Hz, 1H, αCH), 2.95 (s, 3H, NHC CH), 2.04 (dtd, J = 13.9, 6.9, 4.5 Hz, 1H, Val CH), 0.90 (d, J = 6.9 Hz, 3H, Val CH), 0.49 (d, J = 6.9 Hz, 3H, Val CH), −23.84 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 179.0 (C(O)), 149.3 (Ccarbene), 142.1 (Ccarbene), 123.8 (NHC backbone), 123.3 (NHC backbone), 123.1 (NHC backbone), 122.9 (NHC backbone), 61.3 (αC), 38.6 (NHC CH), 37.5 (NHC CH), 36.8 (NHC CH), 35.2 (NHC CH), 31.0 (val CH), 19.3 (Val CH), 16.5 (Val CH). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 502.1789; Found: 502.1777.
3.3. Synthesis of Ir(IMe)2(l-Phg)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-phenylglycine (0.0976 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Phg)(H)(I) (0.1401 g, 66%). Phenyl H NMR signals from both isomers ranged from 7.35–7.13 and overlapped with each other and with the signals for the NHC backbones, while phenyl C NMR signals from both isomers ranged from 128.3–127.1 and overlapped with each other. Analysis for major isomer (55%): H NMR (400 MHz, DMSO-d6, δ): 7.38 (d, J = 2.0 Hz, 1H, NHC backbone), 7.31 (dd, J = 2.2, 0.7 Hz, 2H, NHC backbone), 7.21 (d, J = 1.9 Hz, 1H, NHC backbone), 6.06 (m, 1H, NH), 4.66–4.51 (m, 2H, overlapped αCH and NH), 4.12 (s, 3H, NHC CH), 3.65 (s, 3H, NHC CH), 3.24 (s, 3H, NHC CH), 2.87 (s, 3H, NHC CH), −24.33 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 177.0 (C(O)), 148.4 (Ccarbene), 142.3 (Ccarbene), 140.1 (Ph), 123.3 (NHC backbone), 123.0 (NHC backbone), 122.9 (NHC backbone), 122.8 (NHC backbone), 61.5 (αC), 38.2 (NHC CH), 36.8 (NHC CH), 35.0 (NHC CH). Analysis for minor isomer (45%): H NMR (400 MHz, DMSO-d6, δ): 7.50 (d, J = 2.0 Hz, 1H, NHC backbone), 7.25 (d, J = 1.9 Hz, 2H, NHC backbone), 7.15 (dd, J = 1.9, 0.5 Hz, 1H, NHC backbone), 5.66 (dd, J = 12.3, 6.6 Hz, 1H, NH), 4.78–4.67 (m, 1H, NH), 4.18 (s, 3H, NHC CH), 3.96 (s, 3H, NHC CH), 3.61 (dd, J=10.5, 6.5 Hz, 1H, αCH), 3.33 (s, overlapped with H2O peak, NHC CH), 3.11 (s, 3H, NHC CH), −24.04 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 178.1 (C(O)), 147.8 (Ccarbene), 141.1 (Ccarbene), 141.9 (Ph), 124.0 (NHC backbone), 123.9 (NHC backbone), 123.4 (NHC backbone), 123.2 (NHC backbone), 61.1 (αC), 38.3 (NHC CH), 37.0 (NHC CH), 35.4 (NHC CH). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 536.1632; Found: 536.1636.
3.3.1. Synthesis of Ir(IMe)2(l-Aze)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-azetidine-2-carboxylic acid (0.0653 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Aze)(H)(I) (0.1088 g, 55%). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 486.1476; Found: 486.1476. Due to the presence of many isomers (that could not be separated by column chromatography) and overlapping resonances, the NMR spectra were not assigned for this structure. Additional characterization was not further pursued due to poor catalytic enantioselectivity.
3.3.2. Synthesis of Ir(IMe)2(l-Pro)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-proline (0.0743 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Pro)(H)(I) (0.1861 g, 92%). H NMR (400 MHz, DMSO-d6, δ): 7.42 (d, J = 1.9 Hz, 1H, NHC backbone), 7.35 (d, J = 2.0 Hz, 1H, NHC backbone), 7.31 (d, J = 2.0 Hz, 1H, NHC backbone), 7.25 (d, J = 2.0 Hz, 1H, NHC backbone), 5.97 (dt, J = 10.7, 5.5 Hz, 1H, NH), 4.09 (s, 3H, NHC CH), 3.94 (s, 3H, NHC CH), 3.63 (dd, J = 8.6, 5.5 Hz, 1H, αCH), 3.31 (s, 3H, NHC CH), 3.00 (s, 3H, NHC CH), 2.02 (dq, J = 12.9, 8.0 Hz, 1H, Pro CH), 1.93 (ddd, J = 17.9, 8.9, 4.7 Hz, 1H, Pro CH), 1.77 (dt, J = 13.3, 7.1 Hz, 1H, Pro CH), 1.69 (t, J = 7.5 Hz, 1H, Pro CH), 1.64–1.55 (m, 1H, Pro CH), 1.49 (qd, J = 11.6, 7.2 Hz, 1H, Pro CH), −24.12 (s, 1H, hydride). C NMR (101 MHz, DMSO-d6, δ): 178.9 (C(O)), 150.0 (Ccarbene), 140.2 (Ccarbene), 123.9 (NHC backbone), 123.6 (NHC backbone), 123.3 (NHC backbone), 122.6 (NHC backbone), 60.7 (Pro αCH), 52.4 (Pro CH), 39.8 (NHC CH), 38.3 (NHC CH), 36.8 (NHC CH), 35.4 (NHC CH), 28.9 (Pro CH), 24.1 (Pro CH). HRMS/ESI+ (m/z): Calc. for C15H25O2[193Ir]N5: 500.1632; Found: 500.1624.
3.3.3. Synthesis of Ir(IMe)2(d-Pro)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and d-proline (0.0743 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(d-Pro)(H)(I) (0.1450 g, 72%). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 500.1632; Found: 500.1631. NMR spectra were identical to Ir(IMe)2(l-Pro)(H)(I).
3.3.4. Synthesis of Ir(IMe)2(l-F-Pro)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and cis-4-fluoro-l-proline (0.0859 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-F-Pro)(H)(I) (0.0921 g, 44%). H NMR (400 MHz, DMSO-d6, δ): 7.41 (d, J = 1.9 Hz, 1H, NHC backbone), 7.35 (d, J = 1.9 Hz, 1H, NHC backbone), 7.31 (d, J = 1.9 Hz, 1H, NHC backbone), 7.26 (d, J = 2.0 Hz, 1H, NHC backbone), 6.42 (dt, J = 11.5, 5.4 Hz, 1H, NH), 5.28–4.96 (tm, J = 112.1, 53.9, 1H, FCH), 4.14 (dd, J = 11.4, 5.1 Hz, 1H, αCH), 4.09 (s, 3H, NHC CH), 4.06–3.98 (m, 1H, Pro CH), 3.96 (s, 3H, NHC CH), 3.34 (s, 3H, NHC CH), 3.00 (s, 3H, NHC CH), 2.35–2.15 (m, 2H, proline CH), 1.79 (app dtd, J = 25.4, 13.3, 3.7 1H, Pro CH), −24.19 (s, 1H, hydride). C NMR (400 MHz, DMSO-d6, δ): 149.3 (Ccarbene), 138.9 (Ccarbene), 124.1 (NHC backbone), 123.7 (NHC backbone), 123.4 (NHC backbone), 122.7 (NHC backbone), 93.9 (d, J = 174.8 Hz, Pro CF), 63.4 (Pro αCH), 62.0 (d, J = 18.5 Hz, Pro CH), 38.4 (NHC CH), 37.5 (NHC CH), 36.9 (NHC CH), 35.5 (NHC CH), 35.4 (Pro CH), F NMR (376 MHz, DMSO-d6, δ): −169.18 – −173.96 (m). HRMS/ESI+ (m/z): Calc. for CHFO[193Ir]N: 518.1543; Found: 518.1503.
3.3.5. Synthesis of Ir(IMe)2(l-Pip)(H)(I)
Following the general procedure: [Ir(COD)(IMe)2]I (0.2000 g, 0.3228 mmol) and l-pipecolic acid (0.0834 g, 0.6456 mmol) were reacted in water to give Ir(IMe)2(l-Pip)(H)(I) (0.1359 g, 66%). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 514.1789; Found: 514.1783. Due to the presence of many isomers (that could not be separated by column chromatography) and overlapping resonances, the NMR spectra were not assigned for this structure. Additional characterization was not further pursued due to poor catalytic enantioselectivity.
3.3.6. Synthesis of Ir(IEt)2(l-Pro)(H)(I)
Following the general procedure: [Ir(COD)(IEt)2]I (0.2000 g, 0.2960 mmol) and l-proline (0.0682 g, 0.5920 mmol) were reacted in water to give Ir(IEt)2(l-Pro)(H)(I) (0.1369 g, 68%). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 556.2259; Found: 556.2249. Due to the presence of many isomers (that could not be separated by column chromatography) and overlapping resonances, the NMR spectra were not assigned for this structure. Additional characterization was not further pursued due to poor catalytic enantioselectivity.
3.3.7. Synthesis of Ir(IiPr)2(l-Pro)(H)(Cl)
Following the general procedure: [Ir(COD)(IiPr)2]Cl (0.2000 g, 0.2960 mmol) and l-proline (0.0682 g, 0.5920 mmol) were reacted in water to give Ir(IiPr)2(l-Pro)(H)(Cl) (0.1580 g, 82%). HRMS/ESI+ (m/z): Calc. for CHO[193Ir]N: 612.2885; Found: 612.2887. Due to the presence of many isomers (that could not be separated by column chromatography) and overlapping resonances, the NMR spectra were not assigned for this structure. Additional characterization was not further pursued due to poor catalytic enantioselectivity.