Quarry Residue: Treatment of Industrial Effluent Containing Dye
Abstract
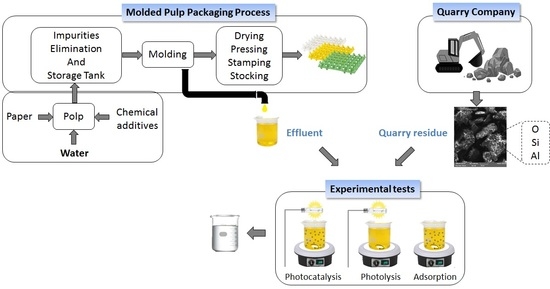
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterizations
2.2. Experimental Tests
PH and Catalyst Concentration
2.3. Industrial Effluent
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts Characterizations
3.2.1. Scanning Electron Microscopy (SEM) Associated with Dispersive Energy Spectroscopy (EDS)
3.2.2. X-ray Diffraction (XRD)
3.2.3. Photoacoustic Spectroscopy (PAS)
3.3. Experimental Tests
3.3.1. PH and Catalyst Concentration Influence
3.3.2. Industrial Effluent—Treatment for One Week
3.4. Characterization of Industrial Effluent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, D.H.; Duarte, J.L.; Tavares, M.G.; Tavares, M.G.; Friedrich, L.C.; Meili, L.; Pimentel, W.R.; Tonholo, J.; Zanta, C.L. Electrochemical degradation and toxicity evaluation of reactive dyes mixture and real textile effluent over DSA® electrodes. Chem. Eng. Process. Process. Intensif. 2020, 153, 107940. [Google Scholar] [CrossRef]
- Ismail, M.; Wu, Z.; Zhang, L.; Ma, J.; Jia, Y.; Hu, Y.; Wang, Y. High—Efficient synergy of piezocatalysis and photocatalysis in bismuth oxychloride nanomaterial for dye decomposition. Chemosphere 2019, 228, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Sadeghfar, F. Efficient adsorption of Azure B onto CNTs/Zn:ZnO@Ni2P-NCs from aqueous solution in the presence of ultrasound wave based on multivariate optimization. J. Ind. Eng. Chem. 2019, 74, 55–62. [Google Scholar] [CrossRef]
- Wu, S.; Xie, Y.; Zhang, X.; Huang, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X. In situ synthesis of adsorptive β-Bi2O3/BiOBr photocatalyst with enhanced degradation efficiency. J. Mater. Res. 2019, 34, 3450–3461. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Josué, T.; Almeida, L.; Lopes, M.; Santos, O.; Lenzi, G. Cr (VI) reduction by photocatalyic process: Nb2O5 an alternative catalyst. J. Environ. Manag. 2020, 268, 110711. [Google Scholar] [CrossRef]
- Lenzi, G.; Favero, C.V.B.; Colpini, L.; Bernabe, H.; Baesso, M.; Specchia, S.; Santos, O. Photocatalytic reduction of Hg(II) on TiO2 and Ag/TiO2 prepared by the sol–gel and impregnation methods. Desalination 2011, 270, 241–247. [Google Scholar] [CrossRef]
- Almeida, L.; Lenzi, G.; Pietrobelli, J.; Santos, O. Caffeine degradation using ZnO and Ag/ZnO under UV and solar radiation. Desalination Water Treat. 2019, 153, 85–94. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Canning, J.; Cook, K.; Johir, A.H.; Zhou, J.L.; Ahmed, M.B. Photocatalysis of 17α-ethynylestradiol and estriol in water using engineered immersible optical fibres and light emitting diodes. J. Water Process. Eng. 2020, 33, 101075. [Google Scholar] [CrossRef]
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of Ofloxacin by Perylene Diimide Supramolecular Nanofiber Sunlight-Driven Photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef]
- Tiwari, D.; Tiwari, A.; Shukla, A.; Kim, D.J.; Yoon, Y.Y.; Lee, S.M. Facile synthesis and characterization of nanocomposite Au0(NPs)/titanium dioxide: Photocatalytic degradation of Alizarin Yellow. J. Ind. Eng. Chem. 2020, 82, 153–163. [Google Scholar] [CrossRef]
- Morais, D.F.; Boaventura, R.A.; Moreira, F.C.; Vilar, V.J. Bromate removal from water intended for human consumption by heterogeneous photocatalysis: Effect of major dissolved water constituents. Chemosphere 2021, 263, 128111. [Google Scholar] [CrossRef]
- Litter, M. Treatment of Chromium, Mercury, Lead, Uranium, and Arsenic in Water by Heterogeneous Photocatalysis, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Zhao, T.; Qian, R.; Zhou, G.; Wang, Y.; Lee, W.I.; Pan, J.H. Mesoporous WO3/TiO2 spheres with tailored surface properties for concurrent solar photocatalysis and membrane filtration. Chemosphere 2021, 263, 128344. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Gonçalves, B.S.; Silva, L.M.; de Souza, T.C.; de Castro, V.G.; Silva, G.G.; Silva, B.C.; Krambrock, K.; Soares, R.B.; Lins, V.F.; Houmard, M.; et al. Solvent effect on the structure and photocatalytic behavior of TiO2-RGO nanocomposites. J. Mater. Res. 2019, 34, 3918–3930. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Ma, L.; Zhang, H. Ni2P/ZnS (CdS) core/shell composites with their photocatalytic performance. J. Mater. Res. 2018, 33, 3580–3588. [Google Scholar] [CrossRef]
- Kako, T.; Yao, W.; Ye, J. Preparation and characterization of visible light sensitive Fe- and Ta-codoped TiO2 photocatalyst. J. Mater. Res. 2010, 25, 110–116. [Google Scholar] [CrossRef]
- Fu, Y.P.; Chang, W.K.; Wang, H.C.; Liu, C.W.; Lin, C.H. Synthesis and characterization of anatase TiO2 nanolayer coating on Ni–Cu–Zn ferrite powders for magnetic photocatalyst. J. Mater. Res. 2010, 25, 134–140. [Google Scholar] [CrossRef]
- Yener, H.B.; Helvacı, Ş.Ş. Effect of synthesis temperature on the structural properties and photocatalytic activity of TiO2/SiO2 composites synthesized using rice husk ash as a SiO2 source. Sep. Purif. Technol. 2015, 140, 84–93. [Google Scholar] [CrossRef]
- Fatimah, I.; Said, A.; Hasanah, U.A. Preparation of TiO2-SiO2 using Rice Husk Ash as Silica Source and The Kinetics Study as Photocatalyst in Methyl Violet Decolorization. Bull. Chem. React. Eng. Catal. 2015, 10, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Haldar, S.K. Igneous rocks. In Introduction to Mineralogy and Petrology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–186. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 2010, 25, 417–425. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.A.; Amare, E. Enhancing the photocatalytic efficiency of ZnO: Defects, heterojunction, and optimization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100336. [Google Scholar] [CrossRef]
- Sa-Nguanprang, S.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Characterization and photocatalysis of visible-light-driven Dy-doped ZnO nanoparticles synthesized by tartaric acid-assisted combustion method. Inorg. Chem. Commun. 2020, 117, 107944. [Google Scholar] [CrossRef]
- Garbarino, G.; Phung, T.K.; Pampararo, G.; Riani, P.; Busca, G. Modification of the properties of γ—Alumina as a support for nickel and molybdate catalysts by addition of silica. Catal. Today 2021. [Google Scholar] [CrossRef]
- Park, J.L.; Canizales, K.A.; Argyle, M.D.; Woodfield, B.F.; Stowers, K.J. The effects of doping alumina with silica in alumina-supported NiO catalysts for oxidative dehydrogenation of ethane. Microporous Mesoporous Mater. 2020, 293, 109799. [Google Scholar] [CrossRef]
- Farjadian, F.; Azadi, S.; Mohammadi-Samani, S.; Ashrafi, H.; Azadi, A. A novel approach to the application of hexagonal mesoporous silica in solid-phase extraction of drugs. Heliyon 2018, 4, e00930. [Google Scholar] [CrossRef] [Green Version]
- Arunmetha, S.; Vinoth, M.; Srither, S.R.; Karthik, A.; Sridharpanday, M.; Suriyaprabha, P.; Manivasakan, R.; Rajendran, V. Study on Production of Silicon Nanoparticles from Quartz Sand for Hybrid Solar Cell Applications. J. Electron. Mater. 2017, 47, 493–502. [Google Scholar] [CrossRef]
- Mangrulkar, P.A.; Kamble, S.P.; Joshi, M.M.; Meshram, J.S.; Labhsetwar, N.K.; Rayalu, S.S. Photocatalytic Degradation of Phenolics by N-Doped Mesoporous Titania under Solar Radiation. Int. J. Photoenergy 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Lansarin, M.A.; Moro, C. Styrene photocatalytic degradation reaction kinetics. J. Braz. Chem. Soc. 2011, 22, 1872–1879. [Google Scholar] [CrossRef] [Green Version]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible Light—Driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Martínez, K.Y.P.; Toso, E.A.V. Planejamento da produção na indústria de embalagens de polpa moldada. Gestão Produção 2014, 23, 649–660. [Google Scholar] [CrossRef]
- Jada, A.; Akbour, R.A. Adsorption and Removal of Organic Dye at Quartz Sand-Water Interface. Oil Gas Sci. Technol. 2014, 69, 405–413. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Process. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Garcia, V.S.; Rosa, J.M.; Borrely, S.I. Toxicity and color reduction of a textile effluent containing reactive red 239 dye by electron beam irradiation. Radiat. Phys. Chem. 2020, 172, 108765. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Juang, R.-S. Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using Platinum—Doped titanate nanomaterials. J. Taiwan Inst. Chem. Eng. 2019, 99, 166–179. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Wang, C.; Chen, X.; Zhu, W.; Zhu, G.; Yao, W.; Zhu, Y. Photocatalysis-self-Fenton system with high-fluent degradation and high mineralization ability. Appl. Catal. B Environ. 2020, 276, 119150. [Google Scholar] [CrossRef]
- Mohammad-Rezaei, R.; Jaymand, M. Graphene quantum dots coated on quartz sand as efficient and low-cost adsorbent for removal of Hg2+ and Pb2+ from aqueous solutions. Environ. Prog. Sustain. Energy 2018, 38, S24–S31. [Google Scholar] [CrossRef]
- Xu, X.; He, J.; Li, Y.; Fang, Z.; Xu, S. Adsorption and Transport of Ciprofloxacin in Quartz Sand at Different pH and Ionic Strength. Open J. Soil Sci. 2014, 04, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Astrath, N.G.C.; Sato, F.; Pedrochi, F.; Medina, A.N.; Bento, A.C.; Baesso, M.L.; Persson, C.; Da Silva, A.F. Band gap energy determination by photoacoustic spectroscopy under continuous light excitation. Appl. Phys. Lett. 2006, 89, 231926. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Shimada, Y.; Suto, T. Potential use of bacteria collected from human hands for textile dye decolorization. Water Resour. Ind. 2018, 20, 46–53. [Google Scholar] [CrossRef]
- Methneni, N.; Morales-González, J.A.; Jaziri, A.; Ben Mansour, H.; Fernandez-Serrano, M. Persistent organic and inorganic pollutants in the effluents from the textile dyeing industries: Ecotoxicology appraisal via a battery of biotests. Environ. Res. 2021, 196, 110956. [Google Scholar] [CrossRef] [PubMed]
- Adamczuk, M.; Pawlik-Skowrońska, B.; Solis, M. Do anthropogenic hydrological alterations in shallow lakes affect the dynamics of plankton? Ecol. Indic. 2020, 114, 106312. [Google Scholar] [CrossRef]
- Mieczan, T.; Rudyk-Leuska, N. Seasonal dynamics of the epibiont food web on Unio tumidus (Philipsson, 1788) in a eutrophic reservoir. Eur. J. Protistol. 2019, 69, 138–150. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, L.N.B.d.; Josué, T.G.; Nogueira, O.H.L.; Dias, D.T.; Tusset, A.M.; Santos, O.A.A.d.; Lenzi, G.G. Quarry Residue: Treatment of Industrial Effluent Containing Dye. Catalysts 2021, 11, 852. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070852
Almeida LNBd, Josué TG, Nogueira OHL, Dias DT, Tusset AM, Santos OAAd, Lenzi GG. Quarry Residue: Treatment of Industrial Effluent Containing Dye. Catalysts. 2021; 11(7):852. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070852
Chicago/Turabian StyleAlmeida, Lariana Negrão Beraldo de, Tatiana Gulminie Josué, Othavio Henrique Lupepsa Nogueira, Daniele Toniolo Dias, Angelo Marcelo Tusset, Onélia Aparecida Andreo dos Santos, and Giane Gonçalves Lenzi. 2021. "Quarry Residue: Treatment of Industrial Effluent Containing Dye" Catalysts 11, no. 7: 852. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070852