Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of of Ag-Co/S Nanoparticles
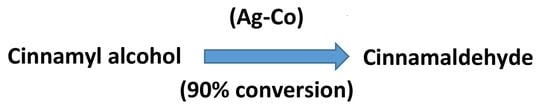
2.2. Oxidation of Cinnamyl Alcohol as a Model Reaction
2.3. Impact of Different Reaction Parameters
2.3.1. Effect of Time
2.3.2. Effect of Temperature
2.3.3. Effect of Stirring Speed
2.3.4. Catalyst Dose Study
2.3.5. Effect of Solvents
2.4. Leaching and Recyclability of Ag-Co/S
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Ag-Co/S Catalyst
3.3. Characterization of Ag-Co/S Catalyst
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaneda, K.; Yamashita, T.; Matsushita, T.; Ebitani, K. Heterogeneous oxidation of allylic and benzylic alcohols catalyzed by Ru-Al-Mg hydrotalcites in the presence of molecular oxygen. J. Org. Chem. 1998, 63, 1750–1751. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, M.E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Alonso González, A. Development of Polymeric Nanocomposites with Enhanced Distribution of Catalytically Active or Bactericide Nanoparticles. Universitat Autònoma de Barcelona: Barcelona, Spain, 2012; pp. 1–75. [Google Scholar]
- Alonso, A.; Davies, G.-L.; Satti, A.; Macanas, J.; Gunko, Y.K.; Munoz, M.; Muraviev, D.N. Intermatrix Synthesis and Characterization of Polymer-Stabilized Functional Metal and Metal Oxide Nanoparticles. In Nanocomposites: In Situ Synthesis of Polymer-Embedded Nanostructures; Wiley: Hoboken, NJ, USA, 2014; pp. 165–194. [Google Scholar]
- Gulari, E.; Güldür, Ç.; Srivannavit, S.; Osuwan, S. Co oxidation by silver cobalt composite oxide. Appl. Catal. A Gen. 1999, 182, 147–163. [Google Scholar] [CrossRef]
- Alonso, A.; Berbel, X.M.; Vigués, N.; Macanás, J.; Muñoz, M.; Mas, J.; Muraviev, D. Characterization of fibrous polymer silver/cobalt nanocomposite with enhanced bactericide activity. Langmuir 2012, 28, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; De Castro, J.; Ticianelli, E.A. Silver-cobalt bimetallic particles for oxygen reduction in alkaline media. J. Power Sources 2006, 161, 806–812. [Google Scholar] [CrossRef]
- Rajesh, D.; Neel, P.I.; Pandurangan, A.; Mahendiran, C. Pd-NiO decorated multiwalled carbon nanotubes supported on reduced graphene oxide as an efficient electrocatalyst for ethanol oxidation in alkaline medium. Appl. Surf. Sci. 2018, 442, 787–796. [Google Scholar] [CrossRef]
- Patila, M.; Chalmpes, N.; Dounousi, E.; Stamatis, H.; Gournis, D. Use of functionalized carbon nanotubes for the development of robust nanobiocatalysts. Methods Enzymol. 2020, 630, 263–301. [Google Scholar] [PubMed]
- Kazici, H.C.; Salman, F.; Caglar, A.; Kivrak, H.; Aktaş, N. Synthesis, characterization, and voltammetric hydrogen peroxide sensing on novel monometallic (Ag, Co/MWCNT) and bimetallic (AgCo/MWCNT) alloy nanoparticles. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 145–151. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Riaz, S.; Manzoor, S.; Tayyeb, A.; Sajid, I.; Naseem, S. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@ AgNPs) and their density-dependent antibacterial activity. R. Soc. Open Sci. 2019, 6, 182135. [Google Scholar] [CrossRef] [Green Version]
- Nyamunda, B.C.; Moyo, M.; Chigondo, F. Catalytic oxidation of cinnamyl alCnOHol to cinnamaldehyde using hydrogen peroxide. IRACST Eng. Sci. Technol. Int. J. 2012, 2, 414–420. [Google Scholar]
- Sadiq, M.; Hussain, R.S.; Zamin, G. Efficiency of iron supported on porous material (prepared from peanut shell) for liquid phase aerobic oxidation of Alcohol. Mod. Res. Catal. 2014. [Google Scholar] [CrossRef] [Green Version]
- Mallat, T.; Bodnar, Z.; Hug, P.; Baiker, A. Selective oxidation of cinnamyl alcohol to cinnamaldehyde with air over Bi-Pt/alumina catalysts. J. Catal. 1995, 153, 131–143. [Google Scholar] [CrossRef]
- Wu, G.; Brett, G.L.; Cao, A.E.; Constantinou, P.; Ellis, S.; Kuhn, G.R.; Hutchings, D.; Bethell, G. AsteriosOxidation of cinnamyl alcoholusing bimetallic Au–Pd/TiO2 catalysts: A deactivation study in a continuous flow packed bed microreactor. Catal. Sci. Technol. 2016, 6, 4749–4758. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.H.; Chen, J.G.; Liu, X.; Liu, Z.W.; Hao, Z.; Xiao, J.; Liu, Z.T. Selective hydrogenation of cinnamaldehyde over Pt and Pd supported on multiwalled carbon nanotubes in a CO2 expanded alcoholic medium. Ind. Eng. Chem. Res. 2012, 51, 11112–11121. [Google Scholar] [CrossRef]
- Sadiq, M.; Saeed, K.; Sadiq, S.; Munir, S.; Khan, M. Liquid phase oxidation of cinnamyl alcohol to cinnamaldehyde using multiwall carbon nanotubes decorated with zinc-manganese oxide nanoparticles. Appl. Catal. A Gen. 2017, 539, 97–103. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Gomez-Lopez, J.; Griffin, K.; Hayes, M. Steric effects in the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol using an Ir/C catalyst. Appl. Catal. A Gen. 2004, 268, 267–274. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Li, L.; Wang, X.; Haller, G.L.; Yang, Y. Carbon nanotube-supported Pt-based bimetallic catalysts prepared by a microwave-assisted polyol reduction method and their catalytic applications in the selective hydrogenation. J. Catal. 2010, 276, 314–326. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sadiq, M.; Khan, M.S.; Iqbal, T.; Ali, M.; Zahoor, M.; Ullah, R.; Almoallim, H.S.; Alharbi, S.A. Functionalized multi walled carbon nanotubes supported copper-titania nanoparticles for oxidation of cinnamyl alcohol under mild reaction conditions. J. King Saud Univ. Sci. 2021, 33, 101273. [Google Scholar] [CrossRef]










| Catalysts | Conv/Sel (%) | Reaction Conditions | References |
|---|---|---|---|
| Bi-Pt/AC | 34/84 | Temp; 60 °C, Solvent; Toluene, Time; 2 h | [14] |
| Fe2O3/AC | 44/89 | Temp; 80 °C, Solvent; Water, Time; 2 h | [15] |
| Bi-Pt/Alumina | >90/>90 | Temp; 100 °C, Detergents; | [16] |
| Au–Pd/TiO2 | 82/64 | Temp; 100 °C, Solvent; Toluene, Time; 7 h | [17] |
| Ag-Co/S | 90/99 | Temp; 75 °C, Solvent; Ethanol, Time; 80 min | The current research |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Khan, M.S.; Khattak, R.; Iqbal, T.; Zekker, I.; Zahoor, M.; Hetta, H.F.; El-Saber Batiha, G.; Alshammari, E.M. Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles. Catalysts 2021, 11, 863. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070863
Iqbal Z, Khan MS, Khattak R, Iqbal T, Zekker I, Zahoor M, Hetta HF, El-Saber Batiha G, Alshammari EM. Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles. Catalysts. 2021; 11(7):863. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070863
Chicago/Turabian StyleIqbal, Zahoor, Muhammad Sufaid Khan, Rozina Khattak, Tausif Iqbal, Ivar Zekker, Muhammad Zahoor, Helal F. Hetta, Gaber El-Saber Batiha, and Eida M. Alshammari. 2021. "Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles" Catalysts 11, no. 7: 863. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070863