Analysis of Photocatalytic Degradation of Phenol with Exfoliated Graphitic Carbon Nitride and Light-Emitting Diodes Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Photocatalyst Synthesis
2.3. Characterization of the Photocatalyst
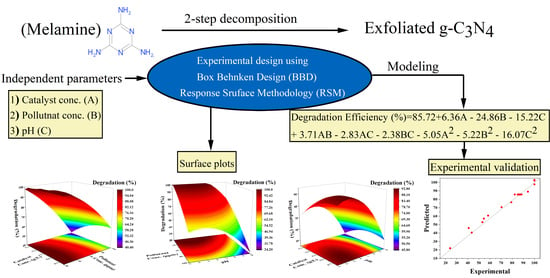
2.4. RSM with Box–Behnken Experimental Design
2.5. Photocatalytic Experiments
2.6. Analytical Techniques
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. Photodegradation Studies
3.3. Response Surface Methodology
3.3.1. Model Equation
3.3.2. Interaction Effects of Independent Operating Parameters
3.3.3. Experimental Validation of RSM Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Facile one-pot synthesis of cerium oxide/sulfur-doped graphitic carbon nitride (g-C3N4) as efficient nanophotocatalysts under visible light irradiation. J. Colloid Interface Sci. 2017, 507, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Eghbali, P.; Metin, O. Sonocatalytic removal of methylene blue from water solution by cobalt ferrite/mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4) nanocomposites: Response surface methodology approach. Environ. Sci. Pollut. Res. Int. 2018, 25, 32140–32155. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef]
- Choquette-Labbé, M.; Shewa, W.; Lalman, J.; Shanmugam, S. Photocatalytic Degradation of Phenol and Phenol Derivatives Using a Nano-TiO2 Catalyst: Integrating Quantitative and Qualitative Factors Using Response Surface Methodology. Water 2014, 6, 1785–1806. [Google Scholar] [CrossRef] [Green Version]
- Yasar Arafath, K.A.; Baskaralingam, P.; Gopinath, S.; Nilavunesan, D.; Sivanesan, S. Degradation of phenol from retting-pond wastewater using anaerobic sludge reactor integrated with photo catalytic treatment. Chem. Phys. Lett. 2019, 734, 136727. [Google Scholar] [CrossRef]
- Hararah, M.A.; Ibrahim, K.A.; Al-Muhtaseb, A.a.H.; Yousef, R.I.; Abu-Surrah, A.; Qatatsheh, A.a. Removal of phenol from aqueous solutions by adsorption onto polymeric adsorbents. J. Appl. Polym. Sci. 2010, 117, 1908–1913. [Google Scholar] [CrossRef]
- Aslam, Z.; Qaiser, M.; Ali, R.; Abbas, A.; Ihsanullah; Zarin, S. Al2O3/MnO2/CNTs nanocomposite: Synthesis, characterization and phenol adsorption. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 591–600. [Google Scholar] [CrossRef]
- Huang, C.H.; Liou, R.M.; Chen, S.H.; Hung, M.Y.; Lai, C.L.; Lai, J.Y. Microbial degradation of phenol in a modified three-stage airlift packing-bed reactor. Water Environ. Res. 2010, 82, 249–258. [Google Scholar] [CrossRef]
- Yavuz, Y.; Savas Koparal, A.; Bakir Ögütveren, Ü. Phenol Removal through Chemical Oxidation using Fenton Reagent. Chem. Eng. Technol. 2007, 30, 583–586. [Google Scholar] [CrossRef]
- Asanjarani, N.; Bagtash, M.; Zolgharnein, J. A comparison between Box–Behnken design and artificial neural network: Modeling of removal of Phenol Red from water solutions by nanocobalt hydroxide. J. Chemom. 2020, 34, e3283. [Google Scholar] [CrossRef]
- Peng, H.; Zou, C.; Wang, C.; Tang, W.; Zhou, J. The effective removal of phenol from aqueous solution via adsorption on CS/beta-CD/CTA multicomponent adsorbent and its application for COD degradation of drilling wastewater. Environ. Sci. Pollut. Res. Int. 2020, 27, 33668–33680. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Nag, S.; Ray, A.K. Degradation of Phenolic Compounds through UV and Visible-Light-Driven Photocatalysis: Technical and Economic Aspects. In Phenolic Compounds—Natural Sources, Importance and Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef] [PubMed]
- Md Rosli, N.I.; Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Mohamed, A.R. Photocatalytic Performance of ZnO/g-C3N4 for Removal of Phenol under Simulated Sunlight Irradiation. J. Environ. Eng. 2018, 144, 04017091. [Google Scholar] [CrossRef]
- Rana, A.G.; Ahmad, W.; Al-Matar, A.; Shawabkeh, R.; Aslam, Z. Synthesis and characterization of Cu-Zn/TiO2 for the photocatalytic conversion of CO2 to methane. Environ. Technol. 2017, 38, 1085–1092. [Google Scholar] [CrossRef]
- Lima, M.J.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Graphitic carbon nitride modified by thermal, chemical and mechanical processes as metal-free photocatalyst for the selective synthesis of benzaldehyde from benzyl alcohol. J. Catal. 2017, 353, 44–53. [Google Scholar] [CrossRef]
- Rana, A.G.; Tasbihi, M.; Schwarze, M.; Minceva, M. Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes. Catalysts 2021, 11, 662. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Abdullah, A.M.; Ahmad, Y.H.; Al-Kandari, S.; AlQaradawi, S.Y.; Mohamed, A.M. An efficient eco advanced oxidation process for phenol mineralization using a 2D/3D nanocomposite photocatalyst and visible light irradiations. Sci. Rep. 2017, 7, 9898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, V.; Ahmed, F.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Acid-treated Fe-doped TiO2 as a high performance photocatalyst used for degradation of phenol under visible light irradiation. J. Environ. Sci. 2019, 83, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Nobijari, L.A.; Schwarze, M.; Tasbihi, M. Photocatalytic Degradation of Phenol Using Photodeposited Pt Nanoparticles on Titania. J. Nanosci. Nanotechnol. 2020, 20, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. In situ fabrication of SnO2/S-doped g-C3N4 nanocomposites and improved visible light driven photodegradation of methylene blue. J. Mol. Liq. 2017, 248, 688–702. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Sulfur-Doped Mesoporous Carbon Nitride Decorated with Cu Particles for Efficient Photocatalytic Degradation under Visible-Light Irradiation. J. Phys. Chem. C 2017, 121, 19239–19253. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable Synthesis of Mesoporous Sulfur-Doped Carbon Nitride Materials for Enhanced Visible Light Photocatalytic Degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Lee, S.C.; Lintang, H.O.; Yuliati, L. A urea precursor to synthesize carbon nitride with mesoporosity for enhanced activity in the photocatalytic removal of phenol. Chem. Asian J. 2012, 7, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-T.; Jia, S.-Y.; Wu, Y.; Wu, S.-H.; Zhang, T.-H.; Han, X. Improved Photochemical Reactivities of Ag2O/g-C3N4 in Phenol Degradation under UV and Visible Light. Ind. Eng. Chem. Res. 2014, 53, 17645–17653. [Google Scholar] [CrossRef]
- Deng, P.; Gan, M.; Zhang, X.; Li, Z.; Hou, Y. Non-noble-metal Ni nanoparticles modified N-doped g-C3N4 for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 30084–30092. [Google Scholar] [CrossRef]
- Hu, J.Y.; Tian, K.; Jiang, H. Improvement of phenol photodegradation efficiency by a combined g-C3N4/Fe(III)/persulfate system. Chemosphere 2016, 148, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Appl. Catal. B Environ. 2013, 136–137, 269–277. [Google Scholar] [CrossRef]
- Sharma, M.; Vaidya, S.; Ganguli, A.K. Enhanced photocatalytic activity of g-C3N4-TiO2 nanocomposites for degradation of Rhodamine B dye. J. Photochem. Photobiol. A Chem. 2017, 335, 287–293. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Moey, H.J.M.; Yusof, N.A. Response surface methodology analysis of the photocatalytic removal of Methylene Blue using bismuth vanadate prepared via polyol route. J. Environ. Sci. 2012, 24, 1694–1701. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Wang, L.; Shi, W. Fabrication, Characterization and Response Surface Method (RSM) Optimization for Tetracycline Photodegration by Bi3.84W0.16O6.24-graphene oxide (BWO-GO). Sci. Rep. 2016, 6, 37466. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, P.; Dou, M.; Wang, J.; Cheng, Y.; Jönsson, P.G.; Zhao, Z. Visible light-driven g-C3N4/m-Ag2Mo2O7composite photocatalysts: Synthesis, enhanced activity and photocatalytic mechanism. RSC Adv. 2014, 4, 51008–51015. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.-T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Kubacka, A.; Luque, R.; Fernández-García, M. Sunlight-Driven Hydrogen Production Using an Annular Flow Photoreactor and g-C3N4-Based Catalysts. ChemPhotoChem 2018, 2, 870–877. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram. Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Facile synthesis of AgNPs modified TiO2@g-C3N4 heterojunction composites with enhanced photocatalytic activity under simulated sunlight. Mater. Res. Bull. 2020, 121, 110641. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, W.; Xu, Y.; Zhou, T.; Xia, M.; Hao, Q. Determination of trace uric acid in serum using porous graphitic carbon nitride (g-C3N4) as a fluorescent probe. Microchim. Acta 2017, 185, 39. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Z.; Sun, Y.; Ho, W.K.; Zhang, H. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Sturini, M.; Speltini, A.; Maraschi, F.; Vinci, G.; Profumo, A.; Pretali, L.; Albini, A.; Malavasi, L. g-C3N4-promoted degradation of ofloxacin antibiotic in natural waters under simulated sunlight. Environ. Sci. Pollut. Res. Int. 2017, 24, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M. Nanocomposites of SnO2 and g-C3N4: Preparation, characterization and photocatalysis under visible LED irradiation. Ceram. Int. 2018, 44, 3837–3846. [Google Scholar] [CrossRef]
- Kumar, M.; Ponselvan, F.I.; Malviya, J.R.; Srivastava, V.C.; Mall, I.D. Treatment of bio-digester effluent by electrocoagulation using iron electrodes. J. Hazard. Mater. 2009, 165, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Leung, C.; Gao, F. A Multi-Parameter Optimization Model for the Evaluation of Shale Gas Recovery Enhancement. Energies 2018, 11, 654. [Google Scholar] [CrossRef] [Green Version]









| Independent Parameters | Symbol | Range and Level | ||
|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | ||
| Catalyst concentration (g/L) | A | 0.25 | 0.5 | 0.75 |
| Phenol initial concentration (ppm) | B | 20 | 60 | 100 |
| pH | C | 3 | 6.5 | 10 |
| Characterization | Bulk g-C3N4 | Exfoliated g-C3N4 | ||
|---|---|---|---|---|
| BET | Surface area | Pore size | Surface area | Pore size |
| 11 m2/g | 1.91 Å | 170 m2/g | 1.96 Å | |
| XRD | Weak peaks (2θ) | Strong peaks (2θ) | Weak peaks (2θ) | Strong peaks (2θ) |
| 13.0° | 27.2° | 13.1° | 27.4° | |
| PL/UV-Vis | Max. absorption | Bandgap | Max. absorption | Bandgap |
| 458 nm | 2.58 eV | 436 nm | 2.68 eV | |
| XPS | C1s peaks | N1s peaks | C1s peaks | N1s peaks |
| 288.2, 284.6, 286.2 and 292.9 eV | 398.5, 399.8, 400.8, 404.1 eV | 287.8, 284.7, 286.2 and 293.5 eV | 397.8, 399.1, 400.1, 403.5 eV | |
| Run | Experimental Conditions | Phenol Degradation Efficiency (%) | |||
|---|---|---|---|---|---|
| Catalyst Concentration (g/L) | Phenol Initial Concentration (ppm) | pH | Experimental | Predicted | |
| 1 | 0.25 | 100 | 6.5 | 43.49 | 44.23 |
| 2 | 0.50 | 60 | 6.5 | 82.25 | 85.72 |
| 3 | 0.25 | 20 | 6.5 | 100 | 93.95 |
| 4 | 0.75 | 60 | 3.0 | 94.09 | 86.18 |
| 5 | 0.75 | 20 | 6.5 | 100.00 | 100.00 |
| 6 | 0.50 | 20 | 10.0 | 79.18 | 74.07 |
| 7 | 0.50 | 20 | 3.0 | 100.00 | 100.00 |
| 8 | 0.50 | 60 | 6.5 | 84.93 | 85.72 |
| 9 | 0.25 | 60 | 10.0 | 40.77 | 43.02 |
| 10 | 0.50 | 60 | 6.5 | 85.94 | 85.72 |
| 11 | 0.50 | 100 | 10.0 | 24.09 | 24.35 |
| 12 | 0.50 | 100 | 3.0 | 54.43 | 54.79 |
| 13 | 0.75 | 60 | 10.0 | 53.15 | 55.74 |
| 14 | 0.25 | 60 | 3.0 | 70.39 | 73.46 |
| 15 | 0.50 | 60 | 6.5 | 88.37 | 85.72 |
| 16 | 0.75 | 100 | 6.5 | 58.31 | 56.95 |
| 17 | 0.50 | 60 | 6.5 | 87.12 | 85.72 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Sequential model sum of squares | ||||||
| Linear | 7118.98 | 3 | 2372.99 | 18.77 | <0.0001 | - |
| 2FI | 109.60 | 3 | 36.53 | 0.238 | 0.8678 | - |
| Quadratic | 1407.83 | 3 | 469.27 | 26.07 | 0.0004 | Suggested |
| Cubic | 104.30 | 3 | 34.76 | 6.41 | 0.0523 | Aliased |
| Lack of fit tests | ||||||
| Linear | 1621.73 | 9 | 180.19 | 33.22 | <0.0021 | - |
| 2FI | 1512.13 | 6 | 252.02 | 46.46 | <0.0012 | - |
| Quadratic | 104.29 | 3 | 34.76 | 6.41 | 0.0523 | Suggested |
| Cubic | 0 | 0 | - | - | - | Aliased |
| Source | Standard deviation | R2 | Adjusted R2 | Predicted R2 | PRESS | - |
| Model summary statistic | ||||||
| Linear | 11.24 | 0.8124 | 0.769 | 0.694 | 2678.06 | - |
| 2FI | 12.38 | 0.8250 | 0.720 | 0.462 | 4712.53 | - |
| Quadratic | 4.24 | 0.9856 | 0.967 | 0.805 | 1702.70 | Suggested |
| Cubic | 2.33 | 0.9975 | 0.999 | - | - | Aliased |
| Factor | Coefficient Estimate | Degree of Freedom | Standard Error | 95% Confidence Interval Low | 95% Confidence Interval Low | F Value | p-Value |
|---|---|---|---|---|---|---|---|
| Intercept | 85.72 | 1 | 1.90 | 81.24 | 90.21 | - | - |
| A | 6.36 | 1 | 1.50 | 2.82 | 9.91 | 17.99 | 0.0038 |
| B | −24.86 | 1 | 1.50 | −28.40 | −21.31 | 274.63 | <0.0001 |
| C | −15.22 | 1 | 1.50 | −18.76 | −11.67 | 102.89 | <0.0001 |
| AB | 3.71 | 1 | 2.12 | −1.31 | 8.72 | 3.05 | 0.1242 |
| AC | −2.83 | 1 | 2.12 | −7.85 | 2.19 | 1.78 | 0.2239 |
| BC | −2.38 | 1 | 2.12 | −7.40 | 2.64 | 1.26 | 0.2989 |
| A2 | −5.05 | 1 | 2.07 | −9.94 | −0.16 | 5.96 | 0.0446 |
| B2 | −5.22 | 1 | 2.07 | −10.11 | −0.33 | 6.38 | 0.0394 |
| C2 | −16.07 | 1 | 2.07 | −20.96 | −11.18 | 60.44 | 0.0001 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Model | 8636.42 | 9 | 959.60 | 53.31 | <0.0001 | Significant |
| Residual | 126.00 | 7 | 18.00 | - | - | - |
| Lack of fit | 104.30 | 3 | 34.77 | 6.41 | 0.0523 | Not Significant |
| Pure error | 21.70 | 4 | 5.42 | - | - | - |
| - | Adjusted R2 = 0.967 | Predicted R2 = 0.810 | Model precision = 24.89 | - | - | |
| - | Std. dev. = 4.24 | Mean = 73.32 | C.V. % = 5.79 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.G.; Minceva, M. Analysis of Photocatalytic Degradation of Phenol with Exfoliated Graphitic Carbon Nitride and Light-Emitting Diodes Using Response Surface Methodology. Catalysts 2021, 11, 898. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080898
Rana AG, Minceva M. Analysis of Photocatalytic Degradation of Phenol with Exfoliated Graphitic Carbon Nitride and Light-Emitting Diodes Using Response Surface Methodology. Catalysts. 2021; 11(8):898. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080898
Chicago/Turabian StyleRana, Adeem Ghaffar, and Mirjana Minceva. 2021. "Analysis of Photocatalytic Degradation of Phenol with Exfoliated Graphitic Carbon Nitride and Light-Emitting Diodes Using Response Surface Methodology" Catalysts 11, no. 8: 898. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080898