Cold Plasma Synthesis and Testing of NiOX-Based Thin-Film Catalysts for CO2 Methanation
Abstract
:1. Introduction
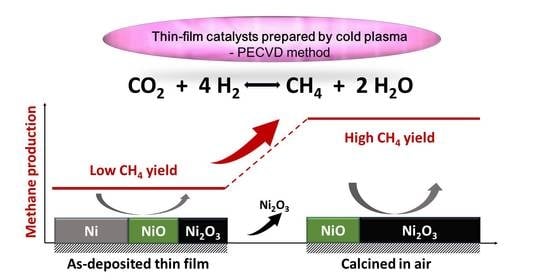
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tyczkowski, J. Cold Plasma produced catalytic materials. In Plasma Science and Technology—Progress in Physical States and Chemical Reactions; Mieno, T., Ed.; InTech Open: Vienna, Austria, 2016; pp. 53–93. [Google Scholar]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.-L.; Liu, C. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Kapica, R.; Balcerzak, J.; Sielski, J. Cold plasma—A promising tool for the production of thin-film nanocatalysts. Catal. Today 2019, 337, 44–54. [Google Scholar] [CrossRef]
- Kołodziej, A.; Łojewska, J.; Tyczkowski, J.; Jodłowski, P.; Redzynia, W.; Iwaniszyn, M.; Zapotoczny, S.; Kuśtrowski, P. Coupled engineering and chemical approach to the design of a catalytic structured reactor for combustion of VOCs: Cobalt oxide catalyst on knitted wire gauzes. Chem. Eng. J. 2012, 200–202, 329–337. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tyczkowski, J.; Balcerzak, J.; Tracz, P. Advances in plasma produced CoOX-based nanocatalysts for CO2 methanation. Catal. Today 2019, 337, 162–170. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tracz, P.; Redzynia, W.; Tyczkowski, J. Plasma deposited novel nanocatalysts for CO2 hydrogenation to methane. J. CO2 Util. 2017, 17, 312–319. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Gac, W.; Zawadzki, W.; Słowik, G.; Sienkiewicz, A.; Kierys, A. Nickel catalysts supported on silica microspheres for CO2 methanation. Microporous Mesoporous Mater. 2018, 272, 79–91. [Google Scholar] [CrossRef]
- Branco, J.B.; Brito, P.E.; Ferreira, A.C. Methanation of CO2 over nickel-lanthanide bimetallic oxides supported on silica. Chem. Eng. J. 2020, 380, 122465. [Google Scholar] [CrossRef]
- Wang, W.; Duong-Viet, C.; Ba, H.; Baaziz, W.; Tuci, G.; Caporali, S.; Nguyen-Dinh, L.; Ersen, O.; Giambastiani, G.; Pham-Huu, C. Nickel nanoparticles decorated nitrogen-doped carbon nanotubes (Ni/N-CNT); a robust catalyst for the efficient and selective CO2 methanation. ACS Appl. Energy Mater. 2019, 2, 1111–1120. [Google Scholar] [CrossRef]
- Ridzuan, N.D.M.; Shaharun, M.S.; Lee, K.M.; Din, I.U.; Puspitasari, P. Influence of nickel loading on reduced graphene oxide-based nickel catalysts for the hydrogenation of carbon dioxide to methane. Catalysts 2020, 10, 471. [Google Scholar] [CrossRef]
- Puga, A.V. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Tada, S.; Nagase, H.; Fujiwara, N.; Kikuchi, R. What are the best active sites for CO2 methanation over Ni/CeO2? Energy Fuels 2021, 35, 5241–5251. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent progresses in constructing the highly efficient Ni based catalysts with advanced low-temperature activity toward CO2 methanation. Front. Chem. 2020, 8, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Pieta, I.S.; Lewalska-Graczyk, A.; Kowalik, P.; Antoniak-Jurak, K.; Krysa, M.; Sroka-Bartnicka, A.; Gajek, A.; Lisowski, W.; Mrdenovic, D.; Pieta, P.; et al. CO2 hydrogenation to methane over Ni-catalysts: The effect of support and vanadia promoting. Catalysts 2021, 11, 433. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K.; Zheng, X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrog. Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, T.; Chen, G.; Li, C.; Dong, D.; Wu, W.; Liu, Q.; Hu, X. Understanding correlation of the interaction between nickel and alumina with the catalytic behaviors in steam reforming and methanation. Fuel 2019, 250, 176–193. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Promoted nickel-based catalysts on modified mesoporous silica support: The role of yttria and magnesia on CO2 methanation. Microporous Mesoporous Mater. 2020, 306, 110455. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Dias, Y.R.; Perez-Lopez, O.W. CO2 conversion to methane using Ni/SiO2 catalysts promoted by Fe, Co and Zn. J. Environ. Chem. Eng. 2021, 9, 104629. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley Company: Weinheim, Germany, 1997. [Google Scholar]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrog. Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Y.; Liu, C.J. Enhanced sulfur resistance of Ni/SiO2 catalyst for methanation via the plasma decomposition of nickel precursor. Phys. Chem. Chem. Phys. 2013, 15, 12132–12138. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Z.; Wang, Y.; Liu, C.J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Int. J. Hydrog. Energy 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Mutz, B.; Gänzler, A.M.; Nachtegaal, M.; Müller, O.; Frahm, R.; Kleist, W.; Grunwaldt, J.D. Surface oxidation of supported Ni particles and its impact on the catalytic performance during dynamically operated methanation of CO2. Catalysts 2017, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Czekaj, I.; Loviat, F.; Raimondi, F.; Wambach, J.; Biollaz, S.; Wokaun, A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl. Catal. A Gen. 2007, 329, 68–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B.; Liu, J.; Chen, G.; Gao, R.; Yao, S.; Li, M.; Zhang, Q.; Gu, L.; Xie, J.; et al. Oxide-modified nickel photocatalysts for the production of hydrocarbons in visible light. Angew. Chem. Int. Ed. 2016, 55, 4215–4219. [Google Scholar] [CrossRef]
- Bi, Q.; Huang, X.; Yin, G.; Chen, T.; Du, X.; Cai, J.; Xu, J.; Liu, Z.; Han, Y.; Huang, F. Cooperative catalysis of nickel and nickel oxide for efficient reduction of CO2 to CH4. ChemCatChem 2019, 11, 1295–1302. [Google Scholar] [CrossRef]
- Huang, X.; Wang, P.; Zhang, Z.; Zhang, S.; Du, X.; Bi, Q.; Huang, F. Efficient conversion of CO2 to methane using thin-layer SiOX matrix anchored nickel catalysts. New J. Chem. 2019, 43, 13217–13224. [Google Scholar] [CrossRef]
- Abu Bakar, W.A.W.; Othman, M.Y.; Ali, R.; Yong, C.K. Nickel oxide based supported catalysts for the in-situ reactions of methanation and desulfurization in the removal of sour gases from simulated natural gas. Catal. Lett. 2009, 128, 127–136. [Google Scholar] [CrossRef]
- Abu Bakar, W.A.W.; Othman, M.Y.; Ali, R.; Yong, C.K.; Toemen, S. The investigation of active sites on nickel oxide based catalysts towards the in-situ reactions of methanation and desulfurization. Mod. Appl. Sci. 2009, 3, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Kreitz, B.; Arias, A.M.; Martin, J.; Weber, A.P.; Turek, T. Spray-dried Ni catalysts with tailored properties for CO2 methanation. Catalysts 2020, 10, 1410. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, L.; Xia, R.; Li, Z. Enhanced low-temperature CO2 methanation activity on plasma-prepared Ni-based catalyst. J. Nat. Gas. Sci. Eng. 2015, 27, 1189–1194. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, Y.; Shi, H.; Yang, F.; Jia, X.; Liu, P.; Ma, X. Preparation of Ni/SiO2 catalyst via novel plasma-induced micro-combustion method. Catal. Today 2019, 337, 28–36. [Google Scholar] [CrossRef]
- Jia, X.; Rui, N.; Zhang, X.; Hu, X.; Liu, C.J. Ni/ZrO2 by dielectric barrier discharge plasma decomposition with improved activity and enhanced coke resistance for CO methanation. Catal. Today 2019, 334, 215–222. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Ryba, M.; Fronczak, M.; Kapica, R.; Sielski, J.; Sitarz, M.; Zając, P.; Łyszczarz, K.; Tyczkowski, J. Enhancing CO2 conversion to CO over plasma-deposited composites based on mixed Co and Fe oxides. Catalysts 2021, 11, 883. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.L.; Liu, Y.; Yang, J.H.; Zong, Z.G. Structural and magnetic properties of hcp and fcc Ni nanoparticles. J. Alloys Compd. 2008, 457, 6–9. [Google Scholar] [CrossRef]
- García-Cerda, L.A.; Bernal-Ramos, K.M.; Montemayor, S.M.; Quevedo-López, M.A.; Betancourt-Galindo, R.; Bueno-Báques, D. Preparation of hcp and fcc Ni and Ni/NiO nanoparticles using a citric acid assisted Pechini-type method. J. Nanomater. 2011, 2011, 162495. [Google Scholar] [CrossRef]
- Zhang, B.; Shang, X.; Jiang, Z.; Song, C.; Maiyalagan, T.; Jiang, Z.J. Atmospheric-pressure plasma jet-induced ultrafast construction of an ultrathin nonstoichiometric nickel oxide layer with mixed Ni3+/Ni2+ ions and rich oxygen defects as an efficient electrocatalyst for oxygen evolution reaction. ACS Appl. Energy Mater. 2021, 4, 5059–5069. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharjee, S.; Chaudhuri, M.G.; Bose, R.S.; Halder, S.; Ghosh, C.K. Synthesis of pure nickel(III) oxide nanoparticles at room temperature for Cr(VI) ion removal. RSC Adv. 2015, 5, 54717–54726. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Ding, M.; Ma, L.; Zhu, X.; Liang, J. Synthesis and electrochemical properties of nickel oxide coated ZnMn2O4 nanocomposites. J. Ceram. Soc. Jpn. 2019, 127, 747–753. [Google Scholar] [CrossRef] [Green Version]
- The Materials Project. Materials Data on Ni2O3 (SG:63) by Materials Project; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014. [Google Scholar] [CrossRef]
- Olesen, S.E.; Andersson, K.J.; Damsgaard, C.D.; Chorkendorff, I. Deactivating carbon formation on a Ni/Al2O3 catalyst under methanation conditions. J. Phys. Chem. C 2017, 121, 15556–15564. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Cattania, M.G.; Villa, P. XPS characterization of Ni and Mo oxides before and after “in situ” treatments. Appl. Surf. Sci. 1993, 70–71, 211–216. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Chan, X.-H.; Jennings, J.R.; Hossain, A.; Yu, K.K.Z.; Wang, Q. Characteristics of p-NiO thin films prepared by spray pyrolysis and their application in CdS-sensitized photocathodes. J. Electrochem. Soc. 2011, 158, H733. [Google Scholar] [CrossRef]
- Chen, C.; Ogino, A.; Wang, X.; Nagatsu, M. Oxygen functionalization of multiwall carbon nanotubes by Ar/H2O plasma treatment. Diam. Relat. Mater. 2011, 20, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yang, W.; Chen, S.; Chu, W. Cerium promoted nano nickel catalysts Ni-Ce/CNTs and Ni-Ce/Al2O3 for CO2 methanation. Integr. Ferroelectr. 2014, 151, 116–125. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Y.; He, H.; Yu, Y.; Yang, Y. A novel W-doped Ni-Mg mixed oxide catalyst for CO2 methanation. Appl. Catal. B Environ. 2016, 196, 108–116. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In Situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-free carbon-based catalyst: An overview and directions for future research. C 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Gardner, S.D.; Singamsetty, C.S.K.; Booth, G.L.; He, G.-R.; Pittman, C.U. Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon 1995, 33, 587–595. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangam, N. C sp2/sp3 hybridisations in carbon nanomaterials—XPS and (X)AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Fidalgo, B.; Zubizarreta, L.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Synthesis of carbon-supported nickel catalysts for the dry reforming of CH4. Fuel Process. Technol. 2010, 91, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yan, Z.; Zhang, Y.; Wang, R.; Luo, S.-Z.; Jing, F.; Chu, W. Carbon nanotubes supported nickel as the highly efficient catalyst for hydrogen production through glycerol steam reforming. ACS Sustain. Chem. Eng. 2018, 6, 14403–14413. [Google Scholar] [CrossRef]







| Catalyst → | ad-NiOX Fresh | ad-NiOX Spent | Ar-NiOX Fresh | Ar-NiOX Spent | air-NiOX Fresh | air-NiOX Spent |
|---|---|---|---|---|---|---|
| Element ↓ | Content [at %] | |||||
| Ni | 30.6 | 33.6 | 1.2 | 1.5 | 31.3 | 33.5 |
| O | 30.2 | 27.6 | 5.2 | 4.2 | 39.6 | 41.7 |
| C | 39.2 | 38.8 | 93.6 | 94.3 | 29.1 | 24.8 |
| Catalyst → | ad-NiOX Fresh | ad-NiOX Spent | Ar-NiOX Fresh | Ar-NiOX Spent | air-NiOX Fresh | air-NiOX Spent |
|---|---|---|---|---|---|---|
| Ni speciation↓ | Content [at %] | |||||
| Ni0 | 40.5 | 69.0 | 32.1 | 63.6 | 0.00 | 55.7 |
| Ni2+ | 29.3 | 20.4 | 44.0 | 36.4 | 32.9 | 28.1 |
| Ni3+ | 30.2 | 10.6 | 23.9 | 0.00 | 67.1 | 16.2 |
| C speciation↓ | Content [at %] | |||||
| C−C/C−H (sp3) | 10.8 | 9.1 | 13.4 | 13.1 | 7.7 | 13.8 |
| C=C (sp2) | 71.2 | 78.5 | 72.4 | 83.4 | 86.4 | 69.9 |
| C−O/C=O | 18.0 | 12.4 | 14.2 | 3.5 | 5.9 | 16.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarek, M.; Kierzkowska-Pawlak, H.; Kapica, R.; Fronczak, M.; Sitarz, M.; Leśniak, M.; Tyczkowski, J. Cold Plasma Synthesis and Testing of NiOX-Based Thin-Film Catalysts for CO2 Methanation. Catalysts 2021, 11, 905. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080905
Smolarek M, Kierzkowska-Pawlak H, Kapica R, Fronczak M, Sitarz M, Leśniak M, Tyczkowski J. Cold Plasma Synthesis and Testing of NiOX-Based Thin-Film Catalysts for CO2 Methanation. Catalysts. 2021; 11(8):905. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080905
Chicago/Turabian StyleSmolarek, Martyna, Hanna Kierzkowska-Pawlak, Ryszard Kapica, Maciej Fronczak, Maciej Sitarz, Magdalena Leśniak, and Jacek Tyczkowski. 2021. "Cold Plasma Synthesis and Testing of NiOX-Based Thin-Film Catalysts for CO2 Methanation" Catalysts 11, no. 8: 905. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080905