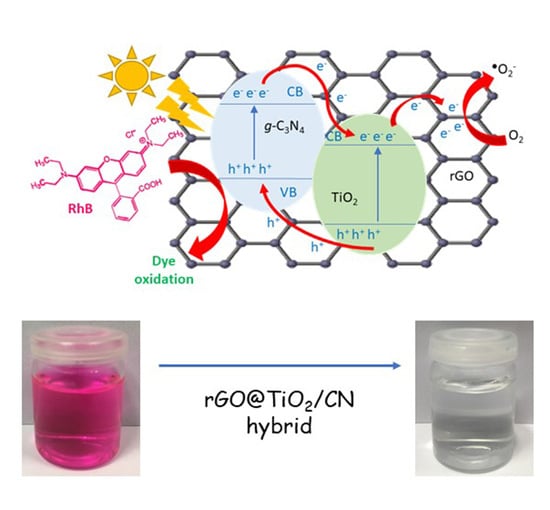
Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of rGO@TiO2/CN Photocatalyst
3.3. Photocatalysts Characterization
3.4. Photolytic, Adsorption and Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathon, B.; Ferreol, M.; Coquery, M.; Choubert, J.M.; Chovelon, J.M.; Miège, C. Direct photodegradation of 36 organic micropollutants under simulated solar radiation: Comparison with free-water surface constructed wetland and influence of chemical structure. J. Hazard. Mater. 2021, 407, 124801. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Unuabonah, I.E. The pathways of microplastics contamination in raw and drinking water. J. Water Process Eng. 2021, 41, 102073. [Google Scholar] [CrossRef]
- Pedrosa, M.; Figueiredo, J.L.; Silva, A.M.T. Graphene-based catalytic membranes for water treatment—A review. J. Environ. Chem. Eng. 2021, 9, 104930. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Lin, P.; Hu, H.; Lv, H.; Ding, Z.; Xu, L.; Qian, D.; Wang, P.; Pan, J.; Li, C.; Cui, C. Hybrid reduced graphene oxide/TiO2/graphitic carbon nitride composites with improved photocatalytic activity for organic pollutant degradation. Appl. Phys. A Mater. Sci. Process. 2018, 124, 510. [Google Scholar] [CrossRef]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-defined Cu2O photocatalysts for solar fuels and chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Hanaor, D.A.H. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Ma, L.; Jia, I.; Guo, X.; Xiang, L. High performance of Pd catalysts on bimodal mesopore for the silica catalytic oxidation of toluene. Chin. J. Catal. 2014, 35, 108–119. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Exploring the synergistic interactions of TiO2, rGO, and g-C3N4 catalyst admixtures in a polystyrene nanocomposite photocatalytic film for wastewater treatment: Unary, binary and ternary systems. J. Environ. Chem. Eng. 2019, 7, 103246. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Hezam, A.; Qahtan, T.F.; Al-Aswad, A.H.; Gondal, M.A.; Drmosh, Q.A. Laser-assisted synthesis of Z-scheme TiO2/rGO/g-C3N4 nanocomposites for highly enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 534, 147578. [Google Scholar] [CrossRef]
- Allen, J.M.; Vincent, T.C.; Richard, K.B. Honeycomb carbon: A Review of Graphene What is graphene ? Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Kovačić, M.; Perović, K.; Papac, J.; Tomić, A.; Matoh, L.; Žener, B.; Brodar, T.; Capan, I.; Surca, A.K.; Kušić, H.; et al. One-Pot Synthesis of Sulfur-Doped TiO2/Reduced Graphene Oxide Composite (S-TiO2/rGO) with Improved Photocatalytic Activity for the Removal of Diclofenac from Water. Materials 2020, 13, 1621. [Google Scholar] [CrossRef] [Green Version]
- Zouzelka, R.; Remzova, M.; Plsek, J.; Brabec, L.; Rathousky, J. Immobilized rGO/TiO2 photocatalyst for decontamination of water. Catalysts 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2019, 357, 630–637. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I.; Petrakis, D.; Vaimakis, T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts 2019, 9, 880. [Google Scholar] [CrossRef] [Green Version]
- Rusek, J.; Paušová, Š.; Praus, P.; Krýsa, J. Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water. Catalysts 2021, 11, 203. [Google Scholar] [CrossRef]
- Starukh, H.; Praus, P. Doping of graphitic carbon nitride with non-metal elements and its applications in photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Fu, L.; Sun, Z.; Ou, M.; Zhao, S.; Chen, Y.; Yu, F.; Wu, Y. A defective g-C3N4/RGO/TiO2composite from hydrogen treatment for enhanced visible-light photocatalytic H2production. Nanoscale 2020, 12, 22030–22035. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Q.; Zhong, Q. One-pot fabrication of mesoporous g-C3N4/NiS co-catalyst counter electrodes for quantum-dot-sensitized solar cells. J. Mater. Sci. 2020, 55, 10712–10724. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.M.; Palanisamy, S. One-Pot Green Synthesis of Graphene Nanosheets Encapsulated Gold Nanoparticles for Sensitive and Selective Detection of Dopamine. Sci. Rep. 2017, 7, 41213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, T.; Tang, J.; Zhang, Y.; Quan, Y.; Gong, X.; Al-Enizi, A.M.; Elzatahry, A.A.; Zhang, L.; Zheng, G. Photoelectrochemical Conversion from Graphitic C3N4 Quantum Dot Decorated Semiconductor Nanowires. ACS Appl. Mater. Interfaces 2016, 8, 12772–12779. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Liu, G.; Cheng, H.M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, E.; Han, J.; Kim, J.-H.; Gurunathan, S. A Novel Biomolecule-Mediated Reduction of Graphene Oxide: A Multifunctional Anti-Cancer Agent. Molecules 2016, 21, 375. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Bellamkonda, S.; Rao, G.R.; Shankar, M.V.; Bahnemann, D.W.; Neppolian, B. Construction of ternary hybrid layered reduced graphene oxide supported g-C3N4-TiO2 nanocomposite and its photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2018, 43, 3892–3904. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Afify, A.D.; Goher, M.E. Preparation and characterization of graphene–TiO2 nanocomposite for enhanced photodegradation of Rhodamine-B dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Maruthamani, D.; Divakar, D.; Kumaravel, M. Enhanced photocatalytic activity of TiO2 by reduced graphene oxide in mineralization of Rhodamine B dye. J. Ind. Eng. Chem. 2015, 30, 33–43. [Google Scholar] [CrossRef]
- Dharwadkar, S.; Yu, L.; Achari, G. Photocatalytic degradation of sulfolane using a led-based photocatalytic treatment system. Catalysts 2021, 11, 624. [Google Scholar] [CrossRef]
- Ćurković, L.; Ljubas, D.; Šegota, S.; Bačić, I. Photocatalytic degradation of Lissamine Green B dye by using nanostructured sol-gel TiO2 films. J. Alloys Compd. 2014, 604, 309–316. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Hinder, S.J.; Pillai, S.C. Photocatalytic properties of g-C3N4-TiO2 heterojunctions under UV and visible light conditions. Materials 2016, 9, 286. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ali Alghamdi, A.; Alanazi, H.S.; Alsyahi, A.A.; Ahmad, N. Photocatalytic Degradation of the Light Sensitive Organic Dyes: Methylene Blue and Rose Bengal by Using Urea Derived g-C3N4/ZnO Nanocomposites. Catalysts 2020, 10, 1457. [Google Scholar] [CrossRef]









| Sample ID | D, nm |
|---|---|
| TiO2 | 7.6 |
| TiO2/rGO | 6.8 |
| TiO2/CN | 7.5 |
| rGO@TiO2/CN | 6.7 |
| SAMPLE ID | k × 10−3, min−1 | η, % |
|---|---|---|
| TiO2 | 6.4 | 54.5 |
| TiO2/rGO | 15.3 | 83.5 |
| TiO2/CN | 13.2 | 79.5 |
| rGO@TiO2/CN | 29.2 | 97.0 |
| pH | k × 10−3, min−1 | η, % |
|---|---|---|
| 3.1 | 14.3 | 73.9 |
| 7.4 | 21.5 | 84.8 |
| 10.7 | 25.0 | 88.8 |
| 12.5 | 44.3 | 97.6 |
| SAMPLE ID | pH | Conductivity, µS/cm | k × 10−3, min−1 | η, % |
|---|---|---|---|---|
| Milli-Q water | 6.18 | 0.380 | 28.7 | 97.2 |
| Alkaline water | 8.81 | 227 | 24.1 | 93.6 |
| Tap water | 7.57 | 492 | 21.3 | 91.9 |
| Parameters | Values |
|---|---|
| Catalyst concentration | 0.5 mg/mL |
| Reaction temperature | 22.0 ± 0.5 °C |
| Radiation source | Solar-like |
| Stirring rate | 350 rpm |
| Distance of light source-reactor | 15 cm |
| Radiation intensity | 64 mW/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091023
Kocijan M, Ćurković L, Radošević T, Podlogar M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts. 2021; 11(9):1023. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091023
Chicago/Turabian StyleKocijan, Martina, Lidija Ćurković, Tina Radošević, and Matejka Podlogar. 2021. "Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation" Catalysts 11, no. 9: 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11091023