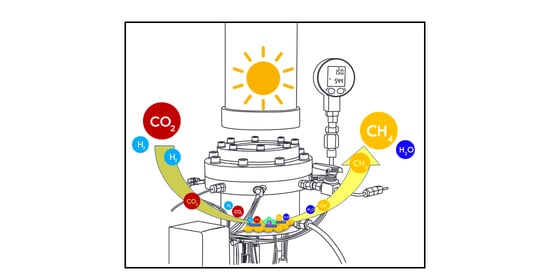
Continuous-Flow Sunlight-Powered CO2 Methanation Catalyzed by γ-Al2O3-Supported Plasmonic Ru Nanorods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Characterization
2.2. CO2 Photomethanation without Conventional Heating
2.3. Thermocatalytic CO2 Methanation in Dark
3. Materials and Methods
3.1. Synthesis of Ru Nanorods Supported on Al2O3
3.2. Characterization
3.3. Gas-Phase Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 Sources for CO2 Utilization by Environmental-Merit-Order Curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Li, Z.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. From Solar Energy to Fuels: Recent Advances in Light-driven C1 Chemistry. Angew. Chem. Int. Ed. 2019, 58, 17528–17551. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, H.-Y.; Liao, J.-F.; Wang, X.-D.; Kuang, D.-B. Enhanced Solar-Driven Gaseous CO2 Conversion by CsPbBr3 Nanocrystal/Pd Nanosheet Schottky-Junction Photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Photocatalytic reduction of CO2 for fuel production: Possibilities and challenges. J. Catal. 2013, 308, 168–175. [Google Scholar] [CrossRef]
- Palmer, C.; Saadi, F.; McFarland, E.W. Technoeconomics of Commodity Chemical Production Using Sunlight. ACS Sustain. Chem. Eng. 2018, 6, 7003–7009. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Cao, E.; Lin, W.; Song, Y.; Liang, W.; Wang, J. Recent advances in surface plasmon-driven catalytic reactions. RSC Adv. 2017, 7, 31189–31203. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Padron, D.; Luque, R.; Munoz-Batista, M.J. Waste-derived Materials: Opportunities in Photocatalysis. Top. Curr. Chem. 2019, 378, 3. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaan, B.; Detz, R.; Meulendijks, N.; Buskens, P. Renewable natural gas as climate-neutral energy carrier? Fuel 2022, 311, 122547. [Google Scholar] [CrossRef]
- Molina, P.M.; Meulendijks, N.; Xu, M.; Verheijen, M.A.; Hartog, T.; Buskens, P.; Sastre, F. Low Temperature Sunlight-Powered Reduction of CO2 to CO Using a Plasmonic Au/TiO2 Nanocatalyst. ChemCatChem 2021, 13, 4507–4513. [Google Scholar] [CrossRef]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent Advances in Plasmonic Nanostructures for Enhanced Photocatalysis and Electrocatalysis. Adv. Mater. 2021, 33, e2000086. [Google Scholar] [CrossRef]
- García-García, I.; Lovell, E.C.; Wong, R.J.; Barrio, V.L.; Scott, J.; Cambra, J.F.; Amal, R. Silver-Based Plasmonic Catalysts for Carbon Dioxide Reduction. ACS Sustain. Chem. Eng. 2020, 8, 1879–1887. [Google Scholar] [CrossRef]
- Naldoni, A.; Riboni, F.; Guler, U.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A.V. Solar-Powered Plasmon-Enhanced Heterogeneous Catalysis. Nanophotonics 2016, 5, 112–133. [Google Scholar] [CrossRef]
- Robatjazi, H.; Zhao, H.; Swearer, D.F.; Hogan, N.J.; Zhou, L.; Alabastri, A.; McClain, M.J.; Nordlander, P.; Halas, N.J. Plasmon-induced selective carbon dioxide conversion on earth-abundant aluminum-cuprous oxide antenna-reactor nanoparticles. Nat. Commun. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Gellé, A.; Moores, A. Plasmonic nanoparticles: Photocatalysts with a bright future. Curr. Opin. Green Sustain. Chem. 2019, 15, 60–66. [Google Scholar] [CrossRef]
- Sastre, F.; Oteri, M.; Corma, A.; García, H. Photocatalytic water gas shift using visible or simulated solar light for the efficient, room-temperature hydrogen generation. Energy Environ. Sci. 2013, 6, 2211–2215. [Google Scholar] [CrossRef]
- Cole, J.R.; Halas, N.J. Optimized plasmonic nanoparticle distributions for solar spectrum harvesting. Appl. Phys. Lett. 2006, 89, 153120. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.G.; Ghuman, K.K.; Jelle, A.A.; Sandhel, A.; Wood, T.E.; Loh, J.Y.Y.; Jia, J.; Perovic, D.; Singh, C.V.; Kherani, N.P.; et al. Enhanced photothermal reduction of gaseous CO2 over silicon photonic crystal supported ruthenium at ambient temperature. Energy Environ. Sci. 2018, 11, 3443–3451. [Google Scholar] [CrossRef]
- O’Brien, P.G.; Sandhel, A.; Wood, T.E.; Jelle, A.A.; Hoch, L.B.; Perovic, D.D.; Mims, C.A.; Ozin, G.A. Photomethanation of Gaseous CO2 over Ru/Silicon Nanowire Catalysts with Visible and Near-Infrared Photons. Adv. Sci. 2014, 1, 1400001. [Google Scholar] [CrossRef]
- Thampi, K.R.; Kiwi, J.; Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Sastre, F.; Puga, A.V.; Liu, L.; Corma, A.; García, H. Complete Photocatalytic Reduction of CO2 to Methane by H2 under Solar Light Irradiation. JACS 2014, 136, 6798–6801. [Google Scholar] [CrossRef] [PubMed]
- Sastre, F.; Versluis, C.; Meulendijks, N.; Rodríguez-Fernández, J.; Sweelssen, J.; Elen, K.; van Bael, M.K.; den Hartog, T.; Verheijen, M.A.; Buskens, P. Sunlight-Fueled, Low-Temperature Ru-Catalyzed Conversion of CO2 and H2 to CH4 with a High Photon-to-Methane Efficiency. ACS Omega 2019, 4, 7369–7377. [Google Scholar] [CrossRef]
- Albero, J.; Garcia, H.; Corma, A. Temperature Dependence of Solar Light Assisted CO2 Reduction on Ni Based Photocatalyst. Top. Catal. 2016, 59, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Sun, W.; Fan, L.; Duchesne, P.N.; Wang, W.; Kubel, C.; Wang, D.; Kumar, S.G.H.; Li, Y.F.; Tavasoli, A.; et al. Nickel@Siloxene catalytic nanosheets for high-performance CO2 methanation. Nat. Commun. 2019, 10, 2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melsheimer, J.; Guo, W.; Ziegler, D.; Wesemann, M.; Schlögl, R. Methanation of carbon dioxide over Ru/Titania at room temperature: Explorations for a photoassisted catalytic reaction. Catal. Lett. 1991, 11, 157–168. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Graphene supported NiO/Ni nanoparticles as efficient photocatalyst for gas phase CO2 reduction with hydrogen. Appl. Catal. B Environ. 2018, 224, 563–571. [Google Scholar] [CrossRef]
- Sastre, F.; Corma, A.; García, H. Visible-Light Photocatalytic Conversion of Carbon Monoxide to Methane by Nickel(II) Oxide. Angew. Chem. Int. Ed. 2013, 52, 12983–12987. [Google Scholar] [CrossRef]
- Barrio, J.; Mateo, D.; Albero, J.; García, H.; Shalom, M. A Heterogeneous Carbon Nitride–Nickel Photocatalyst for Efficient Low-Temperature CO2 Methanation. Adv. Energy Mater. 2019, 9, 1902738. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Titanium-Perovskite-Supported RuO2 Nanoparticles for Photocatalytic CO2 Methanation. Joule 2019, 3, 1949–1962. [Google Scholar] [CrossRef]
- Steeves, T.M.; Esser-Kahn, A.P. Demonstration of the photothermal catalysis of the Sabatier reaction using nickel nanoparticles and solar spectrum light. RSC Adv. 2021, 11, 8394–8397. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem. Int. Ed. 2014, 53, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhang, D.; Su, N.Q.; Yang, W.; Everitt, H.O.; Liu, J. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 2017, 8, 14542. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Photoassisted methanation using Cu2O nanoparticles supported on graphene as a photocatalyst. Energy Environ. Sci. 2017, 10, 2392–2400. [Google Scholar] [CrossRef]
- Grote, R.; Habets, R.; Rohlfs, J.; Sastre, F.; Meulendijks, N.; Xu, M.; Verheijen, M.; Elen, K.; Hardy, A.; van Bael, M.; et al. Collective photothermal effect of Al2O3-supported spheroidalplasmonic Ru nanoparticle catalysts in the sunlight-powered Sabatier reaction. ChemCatChem 2020, 12, 5618–5622. [Google Scholar] [CrossRef]
- Albero, J.; Dominguez, E.; Corma, A.; García, H. Continuous flow photoassisted CO2 methanation. Sustain. Energy Fuels 2017, 1, 1303–1307. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7, 1601657. [Google Scholar] [CrossRef]
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple experimental procedures to distinguish photothermal from hot-carrier processes in plasmonics. Light Sci. Appl. 2020, 9, 108. [Google Scholar] [CrossRef]
- Un, I.-W.; Sivan, Y. The Role of Heat Generation and Fluid Flow in Plasmon-Enhanced Reduction–Oxidation Reactions. ACS Photonics 2021, 8, 1183–1190. [Google Scholar] [CrossRef]
- Dubi, Y.; Un, I.W.; Sivan, Y. Thermal effects—An alternative mechanism for plasmon-assisted photocatalysis. Chem. Sci. 2020, 11, 5017–5027. [Google Scholar] [CrossRef] [Green Version]
- Un, I.-W.; Sivan, Y. Thermal effect in plasmon assisted photocatalyst: A parametric study. In Novel Optical Materials and Applications, Proceedings OSA Advanced Photonics Congress (AP) 2020 (IPR, NP, NOMA, Networks, PVLED, PSC, SPPCom, SOF), Washington, DC, USA, 13–16 July 2020; Optical Society of America: Washington, DC, USA, 2020; p. NoTh3C.3. [Google Scholar]
- Zhou, L.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaiano, J.C.; Stamplecoskie, K. Can Surface Plasmon Fields Provide a New Way to Photosensitize Organic Photoreactions? From Designer Nanoparticles to Custom Applications. J. Phys. Chem. Lett. 2013, 4, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, R.M.; Koopman, W.; Schuetz, R.; Schmid, T.; Liebig, F.; Koetz, J.; Bargheer, M. The importance of plasmonic heating for the plasmon-driven photodimerization of 4-nitrothiophenol. Sci. Rep. 2019, 9, 3060. [Google Scholar] [CrossRef] [PubMed]
- Kamarudheen, R.; Aalbers, G.J.W.; Hamans, R.F.; Kamp, L.P.J.; Baldi, A. Distinguishing Among All Possible Activation Mechanisms of a Plasmon-Driven Chemical Reaction. ACS Energy Lett. 2020, 5, 2605–2613. [Google Scholar] [CrossRef]
- Kamarudheen, R.; Castellanos, G.W.; Kamp, L.P.J.; Clercx, H.J.H.; Baldi, A. Quantifying Photothermal and Hot Charge Carrier Effects in Plasmon-Driven Nanoparticle Syntheses. ACS Nano 2018, 12, 8447–8455. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohlfs, J.; Bossers, K.W.; Meulendijks, N.; Valega Mackenzie, F.; Xu, M.; Verheijen, M.A.; Buskens, P.; Sastre, F. Continuous-Flow Sunlight-Powered CO2 Methanation Catalyzed by γ-Al2O3-Supported Plasmonic Ru Nanorods. Catalysts 2022, 12, 126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020126
Rohlfs J, Bossers KW, Meulendijks N, Valega Mackenzie F, Xu M, Verheijen MA, Buskens P, Sastre F. Continuous-Flow Sunlight-Powered CO2 Methanation Catalyzed by γ-Al2O3-Supported Plasmonic Ru Nanorods. Catalysts. 2022; 12(2):126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020126
Chicago/Turabian StyleRohlfs, Jelle, Koen W. Bossers, Nicole Meulendijks, Fidel Valega Mackenzie, Man Xu, Marcel A. Verheijen, Pascal Buskens, and Francesc Sastre. 2022. "Continuous-Flow Sunlight-Powered CO2 Methanation Catalyzed by γ-Al2O3-Supported Plasmonic Ru Nanorods" Catalysts 12, no. 2: 126. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020126