Cobalt-Containing Nitrogen-Doped Carbon Materials Derived from Saccharides as Efficient Electrocatalysts for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterisation
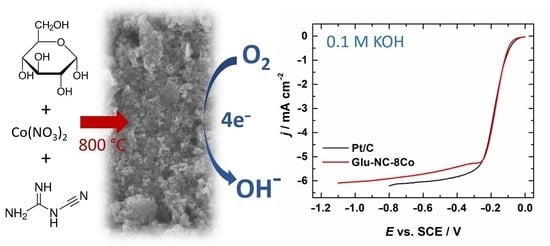
2.2. Electrochemical Characterisation
3. Materials and Methods
3.1. Preparation of Catalyst Materials
3.2. Electrochemical Measurements
3.3. Physicochemical Characterisation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, Y.H.; Liu, S.W.; Priest, C.; Shi, Q.R.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef] [PubMed]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of oxygen reduction on heteroatom-doped nanocarbons and transition metal-nitrogen-carbon catalysts for alkaline membrane fuel cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Alkaline Anion-Exchange Membrane Fuel Cells: Challenges in Electrocatalysis and Interfacial Charge Transfer. Chem. Rev. 2019, 119, 11945–11979. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.T.; Peterson, D.; Ho, D.; Papageorgopoulos, D. Perspective-The Next Decade of AEMFCs: Near-Term Targets to Accelerate Applied R&D. J. Electrochem. Soc. 2020, 167, 084514. [Google Scholar] [CrossRef]
- Liu, D.D.; Tao, L.; Yan, D.F.; Zou, Y.Q.; Wang, S.Y. Recent Advances on Non-precious Metal Porous Carbon-based Electrocatalysts for Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1775–1785. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Osmieri, L.; Pezzolato, L.; Specchia, S. Recent trends on the application of PGM-free catalysts at the cathode of anion exchange membrane fuel cells. Curr. Opin. Electrochem. 2018, 9, 240–256. [Google Scholar] [CrossRef]
- Shen, M.X.; Wei, C.T.; Ai, K.L.; Lu, L.H. Transition metal-nitrogen-carbon nanostructured catalysts for the oxygen reduction reaction: From mechanistic insights to structural optimization. Nano Res. 2017, 10, 1449–1470. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Thorum, M.S.; Hankett, J.M.; Gewirth, A.A. Poisoning the Oxygen Reduction Reaction on Carbon-Supported Fe and Cu Electrocatalysts: Evidence for Metal-Centered Activity. J. Phys. Chem. Lett. 2011, 2, 295–298. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Rähn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional Oxygen Electrocatalysis on Mixed Metal Phthalocyanine-Modified Carbon Nanotubes Prepared via Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef] [PubMed]
- Ratso, S.; Zitolo, A.; Käärik, M.; Merisalu, M.; Kikas, A.; Kisand, V.; Rähn, M.; Paiste, P.; Leis, J.; Sammelselg, V.; et al. Non-precious metal cathodes for anion exchange membrane fuel cells from ball-milled iron and nitrogen doped carbide-derived carbons. Renew. Energy 2021, 167, 800–810. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Douglin, J.C.; Käärik, M.; Kozlova, J.; Paiste, P.; Kikas, A.; Aruväli, J.; Leis, J.; et al. Transition-Metal- and Nitrogen-Doped Carbide-Derived Carbon/Carbon Nanotube Composites as Cathode Catalysts for Anion-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 1920–1931. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Kodali, M.; Chen, Y.C.; Asset, T.; Käärik, M.; Merisalu, M.; Paiste, P.; Aruväli, J.; et al. Cathode Catalysts Based on Cobalt- and Nitrogen-Doped Nanocarbon Composites for Anion Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 5375–5384. [Google Scholar] [CrossRef]
- Lilloja, J.; Mooste, M.; Kibena-Põldsepp, E.; Sarapuu, A.; Zulevi, B.; Kikas, A.; Piirsoo, H.-M.; Tamm, A.; Kisand, V.; Holdcroft, S.; et al. Mesoporous iron-nitrogen co-doped carbon material as cathode catalyst for the anion exchange membrane fuel cell. J. Power Sources Adv. 2021, 8, 100052. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Käärik, M.; Kozlova, J.; Paiste, P.; Kikas, A.; Treshchalov, A.; Leis, J.; Tamm, A.; et al. Transition metal and nitrogen-doped mesoporous carbons as cathode catalysts for anion-exchange membrane fuel cells. Appl. Catal. B 2022, 306, 121113. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-Põldsepp, E.; Ratso, S.; Kook, M.; Sougrati, M.T.; Käärik, M.; Merisalu, M.; Aruväli, J.; Paiste, P.; Treshchalov, A.; et al. Iron- and Nitrogen-Doped Graphene-Based Catalysts for Fuel Cell Applications. ChemElectroChem 2020, 7, 1739–1747. [Google Scholar] [CrossRef]
- Mooste, M.; Tkesheliadze, T.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Aruväli, J.; Zagal, J.H.; Kannan, A.M.; et al. Transition metal phthalocyanine-modified shungite-based cathode catalysts for alkaline membrane fuel cell. Int. J. Hydrogen Energy 2021, 46, 4365–4377. [Google Scholar] [CrossRef]
- Kisand, K.; Sarapuu, A.; Peikolainen, A.L.; Seemen, H.; Kook, M.; Käärik, M.; Leis, J.; Sammelselg, V.; Tammeveski, K. Oxygen Reduction on Fe- and Co-Containing Nitrogen-Doped Nanocarbons. ChemElectroChem 2018, 5, 2002–2009. [Google Scholar] [CrossRef]
- Kisand, K.; Sarapuu, A.; Danilian, D.; Kikas, A.; Kisand, V.; Rähn, M.; Treshchalov, A.; Käärik, M.; Merisalu, M.; Paiste, P.; et al. Transition metal-containing nitrogen-doped nanocarbon catalysts derived from 5-methylresorcinol for anion exchange membrane fuel cell application. J. Colloid Interface Sci. 2021, 584, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kisand, K.; Sarapuu, A.; Kikas, A.; Kisand, V.; Rähn, M.; Treshchalov, A.; Käärik, M.; Piirsoo, H.M.; Aruväli, J.; Paiste, P.; et al. Bifunctional multi-metallic nitrogen-doped nanocarbon catalysts derived from 5-methylresorcinol. Electrochem. Comm. 2021, 124, 106932. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Vassiljeva, V.; Kikas, A.; Käärik, M.; Kozlova, J.; Kisand, V.; Külaviir, M.; Cavaliere, S.; Leis, J.; et al. Electrospun Polyacrylonitrile-Derived Co or Fe Containing Nanofibre Catalysts for Oxygen Reduction Reaction at the Alkaline Membrane Fuel Cell Cathode. ChemCatChem 2020, 12, 4568–4581. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of Multitudinous Active Sites for Oxygen Reduction Reaction in Transition Metal-Nitrogen-Carbon Electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Kreek, K.; Sarapuu, A.; Samolberg, L.; Joost, U.; Mikli, V.; Koel, M.; Tammeveski, K. Cobalt-Containing Nitrogen-Doped Carbon Aerogels as Efficient Electrocatalysts for the Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 2079–2088. [Google Scholar] [CrossRef]
- Sokka, A.; Mooste, M.; Käärik, M.; Gudkova, V.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Paiste, P.; et al. Iron and cobalt containing electrospun carbon nanofibre-based cathode catalysts for anion exchange membrane fuel cell. Int. J. Hydrogen Energy 2021, 46, 31275–31287. [Google Scholar] [CrossRef]
- Jiang, M.H.; Yu, X.F.; Yang, H.Q.; Chen, S.L. Optimization Strategies of Preparation of Biomass-Derived Carbon Electrocatalyst for Boosting Oxygen Reduction Reaction: A Minireview. Catalysts 2020, 10, 21472. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Li, Q.; Xu, D.; Ou, X.; Yan, F. Nitrogen-Doped Graphitic Porous Carbon Nanosheets Derived from In Situ Formed g-C3N4 Templates for the Oxygen Reduction Reaction. Chem. Asian J. 2017, 12, 1816–1823. [Google Scholar] [CrossRef]
- Zhen, Z.H.; Jiang, Z.Q.; Tian, X.N.; Zhou, L.S.; Deng, B.L.; Chen, B.H.; Jiang, Z.J. Core@shell structured Co-CoO@NC nanoparticles supported on nitrogen doped carbon with high catalytic activity for oxygen reduction reaction. RSC Adv. 2018, 8, 14462–14472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juvanen, S.; Sarapuu, A.; Vlassov, S.; Kook, M.; Kisand, V.; Kaarik, M.; Treshchalov, A.; Aruvali, J.; Kozlova, J.; Tamm, A.; et al. Iron-Containing Nitrogen-Doped Carbon Nanomaterials Prepared via NaCl Template as Efficient Electrocatalysts for the Oxygen Reduction Reaction. ChemElectroChem 2021, 8, 2288–2297. [Google Scholar] [CrossRef]
- Eissa, A.A.; Peera, S.G.; Kim, N.H.; Lee, J.H. g-C3N4 templated synthesis of the Fe3C@NSC electrocatalyst enriched with Fe-N-x active sites for efficient oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 16920–16936. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, W.T.; Cao, F.F.; Xiao, Z.D.; Zheng, X.S. N-doped graphene coupled with Co nanoparticles as an efficient electrocatalyst for oxygen reduction in alkaline media. J. Power Sources 2016, 302, 114–125. [Google Scholar] [CrossRef]
- Pan, F.P.; Jin, J.T.; Fu, X.G.; Liu, Q.; Zhang, J.Y. Advanced Oxygen Reduction Electrocatalyst Based on Nitrogen-Doped Graphene Derived from Edible Sugar and Urea. ACS Appl. Mater. Interfaces 2013, 5, 11108–11114. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, Y.X.; Zhao, Q.P.; Pan, F.P.; Zhang, B.; Zhang, J.Y. Direct Synthesis of Nitrogen-Doped Carbon Nanosheets with High Surface Area and Excellent Oxygen Reduction Performance. Langmuir 2014, 30, 8238–8245. [Google Scholar] [CrossRef]
- Enterria, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Li, X.H.; Kurasch, S.; Kaiser, U.; Antonietti, M. Synthesis of Monolayer-Patched Graphene from Glucose. Angew. Chem. Int. Ed. 2012, 51, 9689–9692. [Google Scholar] [CrossRef]
- Tian, Y.H.; Xu, L.; Bao, J.; Qian, J.C.; Su, H.N.; Li, H.M.; Gu, H.D.; Yan, C.; Li, H.N. Hollow cobalt oxide nanoparticles embedded in nitrogen-doped carbon nanosheets as an efficient bifunctional catalyst for Zn-air battery. J. Energy Chem. 2019, 33, 59–66. [Google Scholar] [CrossRef]
- Asset, T.; Atanassov, P. Iron-Nitrogen-Carbon Catalysts for Proton Exchange Membrane Fuel Cells. Joule 2020, 4, 33–44. [Google Scholar] [CrossRef]
- Deng, D.H.; Yu, L.; Chen, X.Q.; Wang, G.X.; Jin, L.; Pan, X.L.; Deng, J.; Sun, G.Q.; Bao, X.H. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.J.; Wang, J.Q.; Hu, J.S.; Wei, Z.D.; Wan, L.J. Understanding the High Activity of Fe-N-C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe-N-x. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen reduction reaction mechanism and kinetics on M-N(x)C(y)and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 2021, 25, 45–56. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.W.; Zhuang, X.D.; Bruller, S.; Feng, X.L.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldie, S.J.; Jiang, S.; Coleman, K.S. Cobalt nanoparticle catalysed graphitization and the effect of metal precursor decomposition temperature. Mater. Adv. 2021, 2, 3353–3361. [Google Scholar] [CrossRef]
- Jia, Q.; Ramaswamy, N.; Tylus, U.; Strickland, K.; Li, J.; Serov, A.; Artyushkova, K.; Atanassov, P.; Anibal, J.; Gumeci, C.; et al. Spectroscopic insights into the nature of active sites in iron–nitrogen–carbon electrocatalysts for oxygen reduction in acid. Nano Energy 2016, 29, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Ratso, S.; Kruusenberg, I.; Sarapuu, A.; Kook, M.; Rauwel, P.; Saar, R.; Aruvali, J.; Tammeveski, K. Electrocatalysis of oxygen reduction on iron- and cobalt-containing nitrogen-doped carbon nanotubes in acid media. Electrochim. Acta 2016, 218, 303–310. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Mineva, T.; Matanovic, I.; Atanassov, P.; Sougrati, M.T.; Stievano, L.; Clemancey, M.; Kochem, A.; Latour, J.M.; Jaouen, F. Understanding Active Sites in Pyrolyzed Fe-N-C Catalysts for Fuel Cell Cathodes by Bridging Density Functional Theory Calculations and Fe-57 Mossbauer Spectroscopy. ACS Catal. 2019, 9, 9359–9371. [Google Scholar] [CrossRef]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.K.; Jia, Q.Y.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017, 8, 957. [Google Scholar] [CrossRef]
- Zagal, J.H.; Specchia, S.; Atanassov, P. Mapping transition metal-MN4 macrocyclic complex catalysts performance for the critical reactivity descriptors. Curr. Opin. Electrochem. 2021, 27, 100683. [Google Scholar] [CrossRef]
- He, Y.H.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.Y.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, L.; Xu, T.; Wei, H.H.; Zhang, R.Y.; Zhang, X.Y.; Ge, B.H.; Lei, M.; Ma, J.Y.; Liu, L.M.; et al. −60 °C solution synthesis of atomically dispersed cobalt electrocatalyst with superior performance. Nat. Commun. 2019, 10, 606. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zeng, R.; Xiong, Y.; DiSalvo, F.J.; Abruna, H.D. Cobalt-Based Nitride-Core Oxide-Shell Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2019, 141, 19241–19245. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.Y.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating Oxygen Reduction Active Sites in Pyrolyzed Metal-Nitrogen Coordinated Non-Precious-Metal Electrocatalyst Systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef]






| Catalyst | E1/2 (V vs. SCE) | Eonset (V vs. SCE) | n (at −1.1 V) |
|---|---|---|---|
| Glu-NC | −0.40 | −0.21 | 3.2 |
| Glu-NC-Co | −0.24 | −0.14 | 4.0 |
| Glu-NC-4Co | −0.22 | −0.11 | 4.0 |
| Glu-NC-8Co | −0.18 | −0.06 | 4.3 |
| Glu-NC-16Co | −0.18 | −0.04 | 4.1 |
| Glu-NC-8Co-at | −0.20 | −0.07 | 4.1 |
| Xylan-NC-8Co | −0.19 | −0.07 | 4.0 |
| Xylose-NC-8Co | −0.20 | −0.07 | 4.1 |
| CD-NC-8Co | −0.19 | −0.07 | 4.2 |
| Pt/C | −0.18 | −0.04 | 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veske, K.; Sarapuu, A.; Käärik, M.; Kikas, A.; Kisand, V.; Piirsoo, H.-M.; Treshchalov, A.; Leis, J.; Tamm, A.; Tammeveski, K. Cobalt-Containing Nitrogen-Doped Carbon Materials Derived from Saccharides as Efficient Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2022, 12, 568. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050568
Veske K, Sarapuu A, Käärik M, Kikas A, Kisand V, Piirsoo H-M, Treshchalov A, Leis J, Tamm A, Tammeveski K. Cobalt-Containing Nitrogen-Doped Carbon Materials Derived from Saccharides as Efficient Electrocatalysts for Oxygen Reduction Reaction. Catalysts. 2022; 12(5):568. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050568
Chicago/Turabian StyleVeske, Kaidi, Ave Sarapuu, Maike Käärik, Arvo Kikas, Vambola Kisand, Helle-Mai Piirsoo, Alexey Treshchalov, Jaan Leis, Aile Tamm, and Kaido Tammeveski. 2022. "Cobalt-Containing Nitrogen-Doped Carbon Materials Derived from Saccharides as Efficient Electrocatalysts for Oxygen Reduction Reaction" Catalysts 12, no. 5: 568. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050568