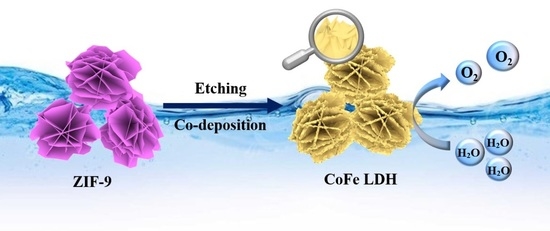
Facile Synthesis of Sulfate-Intercalated CoFe LDH Nanosheets Derived from Two-Dimensional ZIF-9(III) for Promoted Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Procedure and Methods
3.1. Materials
3.2. Synthesis of Compounds
3.2.1. Preparation of 2D ZIF-9(III)
3.2.2. Synthesis of Hierarchical CoFe LDH Nanosheets
3.3. Physicochemical Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, D.; Li, P.; Lin, X.; Mckinley, A.; Kuang, Y.; Liu, W.; Lin, W.-F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Li, J.; Jiang, S.; Shao, M.; Wei, M. Host-Guest Engineering of Layered Double Hydroxides towards Efficient Oxygen Evolution Reaction: Recent Advances and Perspectives. Catalysts 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yu, F.; Yuen, M.-F.; Wang, C. Two-dimensional layered double hydroxides as a platform for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 9389–9430. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhong, H.; Jiang, Y.; Guo, F.; Alonso-Vante, N.; Feng, Y. Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions. Catalysts 2021, 11, 1394. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Sankar, S.S.; Kumaravel, S.; Madhu, R.; Kundu, S. A vast exploration of improvising synthetic strategies for enhancing the OER kinetics of LDH structures: A review. J. Mater. Chem. A 2021, 9, 1314–1352. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Yang, L.; Gao, Q. Recent advances of two-dimensional CoFe layered-double-hydroxides for electrocatalytic water oxidation. Chin. Chem. Lett. 2022, 33, 2845–2855. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt–Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Hu, Y.; Guan, M.; Xu, L.; Li, H.; Bao, J.; Li, H. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B 2020, 272, 118959. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Feng, L.; Li, A.; Li, Y.; Liu, J.; Wang, L.; Huang, L.; Wang, Y.; Ge, X. A Highly Active CoFe Layered Double Hydroxide for Water Splitting. ChemPlusChem 2017, 82, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Li, Z.; Dai, X.; Yin, X.; Gan, Y.; Yang, Z.; Wu, B.; Ren, Z.; Cao, Y.; Song, W. Interfacial electronic modulation on heterostructured NiSe@CoFe LDH nanoarrays for enhancing oxygen evolution reaction and water splitting by facilitating the deprotonation of OH to O. Chem. Eng. J. 2022, 431, 134080. [Google Scholar] [CrossRef]
- Dang, L.; Liang, H.; Zhuo, J.; Lamb, B.K.; Sheng, H.; Yang, Y.; Jin, S. Direct Synthesis and Anion Exchange of Noncarbonate-Intercalated NiFe-Layered Double Hydroxides and the Influence on Electrocatalysis. Chem. Mater. 2018, 30, 4321–4330. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Müller, A.M. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hao, Y.; Zhang, G.; Lu, Z.; Han, S.; Li, Y.; Sun, X. Room-temperature synthetic NiFe layered double hydroxide with different anions intercalation as an excellent oxygen evolution catalyst. RSC Adv. 2015, 5, 55131–55135. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Xue, J.-Y.; Zhao, Z.-Y.; Li, C.; Li, F.-L.; Cao, C.; Niu, Z.; Gu, H.-W.; Lang, J.-P. Ultrathin sulfate-intercalated NiFe-layered double hydroxide nanosheets for efficient electrocatalytic oxygen evolution. RSC Adv. 2020, 10, 12145–12150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wang, J.; Jin, W.; Wu, Z. Recent development of two-dimensional metal–organic framework derived electrocatalysts for hydrogen and oxygen electrocatalysis. Nanoscale 2020, 12, 18497–18522. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, F.; Chen, W.; Wang, C.; Cai, D. Facile synthesis of hollow Co3O4-embedded carbon/reduced graphene oxides nanocomposites for use as efficient electrocatalysts in oxygen evolution reaction. Electrochim. Acta 2019, 300, 123–130. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Yuan, D.; Qian, S.; Cai, D.; Jiang, J.; Zhang, S. Tailoring the nanostructure and electronic configuration of metal phosphides for efficient electrocatalytic oxygen evolution reactions. Nano Energy 2020, 69, 104453. [Google Scholar] [CrossRef]
- Liu, M.; Kong, L.; Wang, X.; He, J.; Bu, X.-H. Engineering Bimetal Synergistic Electrocatalysts Based on Metal–Organic Frameworks for Efficient Oxygen Evolution. Small 2019, 15, 1903410. [Google Scholar] [CrossRef] [PubMed]
- Rinawati, M.; Wang, Y.-X.; Chen, K.-Y.; Yeh, M.-H. Designing a spontaneously deriving NiFe-LDH from bimetallic MOF-74 as an electrocatalyst for oxygen evolution reaction in alkaline solution. Chem. Eng. J. 2021, 423, 130204. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Chen, Y.; Lu, X.F.; Gao, S.; Lou, X.W. Designed Formation of Double-Shelled Ni–Fe Layered-Double-Hydroxide Nanocages for Efficient Oxygen Evolution Reaction. Adv. Mater. 2020, 32, 1906432. [Google Scholar] [CrossRef]
- Li, J.-G.; Sun, H.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Li, Y.; Wang, C. Metal–Organic Framework-Derived Hierarchical (Co,Ni)Se2@NiFe LDH Hollow Nanocages for Enhanced Oxygen Evolution. ACS Appl. Mater. Interfaces 2019, 11, 8106–8114. [Google Scholar] [CrossRef]
- Hu, L.; Xiao, R.; Wang, X.; Wang, X.; Wang, C.; Wen, J.; Gu, W.; Zhu, C. MXene-induced electronic optimization of metal-organic framework-derived CoFe LDH nanosheet arrays for efficient oxygen evolution. Appl. Catal. B 2021, 298, 120599. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; He, H.; Chen, Y.; Gao, Z.; Ma, N.; Wang, B.; Zheng, L.; Li, R.; Wei, Y.; et al. Interface construction of NiCo LDH/NiCoS based on the 2D ultrathin nanosheet towards oxygen evolution reaction. Nano Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.; Su, S.; Wang, H.; Cai, D. Synthesis of ZIF-9(III)/Co LDH layered composite from ZIF-9(I) based on controllable phase transition for enhanced electrocatalytic oxygen evolution reaction. Chem. Eng. J. 2021, 414, 128784. [Google Scholar] [CrossRef]
- Zhao, P.; Lampronti, G.I.; Lloyd, G.O.; Wharmby, M.T.; Facq, S.; Cheetham, A.K.; Redfern, S. A T. Phase Transitions in Zeolitic Imidazolate Framework 7: The Importance of Framework Flexibility and Guest-Induced Instability. Chem. Mater. 2014, 26, 1767–1769. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Peng, C.-K.; Bu, L.; Chiang, C.-L.; Tian, K.; Zhao, Y.; Zhao, J.; Lin, Y.-G.; Lee, J.-M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 16, 2002426. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Mansournia, M. Cost-effective fabrication of thermal- and chemical-stable ZIF-9 nanocrystals at ammonia atmosphere. J. Phys. Chem. Solids 2017, 111, 12–17. [Google Scholar] [CrossRef]
- Wang, H.; Jin, Z. Rational design W-doped Co-ZIF-9 based Co3S4 composite photocatalyst for efficient visible-light-driven photocatalytic H2 evolution. Sustain. Energy Fuels 2019, 3, 173–183. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Zhu, K.; Gao, P.; Zhu, L. Mechanisms for light-driven evolution of environmentally persistent free radicals and photolytic degradation of PAHs on Fe(III)-montmorillonite surface. J. Hazard. Mater. 2019, 362, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Carja, G.; Grosu, E.F.; Mureseanu, M.; Lutic, D. A family of solar light responsive photocatalysts obtained using Zn2+ Me3+ (Me = Al/Ga) LDHs doped with Ga2O3 and In2O3 and their derived mixed oxides: A case study of phenol/4-nitrophenol decomposition. Catal. Sci. Technol. 2017, 7, 5402–5412. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis and characterization of Hydrotalcite like Mg–Al–SO4-LDH. Mater. Lett. 2016, 165, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Jayaramulu, K.; Masa, J.; Morales, D.M.; Tomanec, O.; Ranc, V.; Petr, M.; Wilde, P.; Chen, Y.-T.; Zboril, R.; Schuhmann, W.; et al. Ultrathin 2D Cobalt Zeolite-Imidazole Framework Nanosheets for Electrocatalytic Oxygen Evolution. Adv. Sci. 2018, 5, 1801029. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Chen, J.; Wang, W.; Liao, J.; Dong, J.; Chee, M.O.L.; Wang, N.; Dong, P.; Ajayan, P.M.; Gao, S.; et al. Etching-Doping Sedimentation Equilibrium Strategy: Accelerating Kinetics on Hollow Rh-Doped CoFe-Layered Double Hydroxides for Water Splitting. Adv. Funct. Mater. 2020, 30, 2003556. [Google Scholar] [CrossRef]
- Shi, Y.; Du, W.; Zhou, W.; Wang, C.; Lu, S.; Lu, S.; Zhang, B. Unveiling the Promotion of Surface-Adsorbed Chalcogenate on the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 22470–22474. [Google Scholar] [CrossRef]
- Yang, F.; Sliozberg, K.; Sinev, I.; Antoni, H.; Bähr, A.; Ollegott, K.; Xia, W.; Masa, J.; Grünert, W.; Cuenya, B.R.; et al. Synergistic Effect of Cobalt and Iron in Layered Double Hydroxide Catalysts for the Oxygen Evolution Reaction. ChemSusChem 2017, 10, 156–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Jiang, X.; Lu, W.; Xing, Y. Self-supported hierarchical CoFe-LDH/NiCo2O4/NF core-shell nanowire arrays as an effective electrocatalyst for oxygen evolution reaction. J. Alloys Compd. 2020, 818, 153345. [Google Scholar] [CrossRef]
- Liu, H.; Zha, M.; Liu, Z.; Tian, J.; Hu, G.; Feng, L. Synergistically boosting the oxygen evolution reaction of an Fe-MOF via Ni doping and fluorination. Chem. Commun. 2020, 56, 7889–7892. [Google Scholar] [CrossRef]
- Li, F.; Tian, Y.; Su, S.; Wang, C.; Li, D.-S.; Cai, D.; Zhang, S. Theoretical and experimental exploration of tri-metallic organic frameworks (t-MOFs) for efficient electrocatalytic oxygen evolution reaction. Appl. Catal. B 2021, 299, 120665. [Google Scholar] [CrossRef]
- Battiato, S.; Urso, M.; Cosentino, S.; Pellegrino, A.L.; Mirabella, S.; Terrasi, A. Optimization of Oxygen Evolution Reaction with Electroless Deposited Ni–P Catalytic Nanocoating. Nanomaterials 2021, 11, 3010. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.B.; Enman, L.J.; Batchellor, A.S.; Cosby, M.R.; Vise, A.E.; Trang, C.D.M.; Boettcher, S.W. Measurement Techniques for the Study of Thin Film Heterogeneous Water Oxidation Electrocatalysts. Chem. Mater. 2017, 29, 120–140. [Google Scholar] [CrossRef]
- Wang, C.-P.; Feng, Y.; Sun, H.; Wag, Y.; Yin, J.; Yao, Z.; Bu, X.-H.; Zhu, J. Self-Optimized Metal–Organic Framework Electrocatalysts with Structural Stability and High Current Tolerance for Water Oxidation. ACS Catal. 2021, 11, 7132–7143. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Chen, W.; Cai, Y.; Zhang, S.; Wang, D.; Cai, D. Facile Synthesis of Sulfate-Intercalated CoFe LDH Nanosheets Derived from Two-Dimensional ZIF-9(III) for Promoted Oxygen Evolution Reaction. Catalysts 2022, 12, 688. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070688
Xiao G, Chen W, Cai Y, Zhang S, Wang D, Cai D. Facile Synthesis of Sulfate-Intercalated CoFe LDH Nanosheets Derived from Two-Dimensional ZIF-9(III) for Promoted Oxygen Evolution Reaction. Catalysts. 2022; 12(7):688. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070688
Chicago/Turabian StyleXiao, Guolei, Weibin Chen, Yaming Cai, Shifan Zhang, Di Wang, and Dandan Cai. 2022. "Facile Synthesis of Sulfate-Intercalated CoFe LDH Nanosheets Derived from Two-Dimensional ZIF-9(III) for Promoted Oxygen Evolution Reaction" Catalysts 12, no. 7: 688. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070688