Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants
Abstract
:1. Introduction
2. Results and Discussion
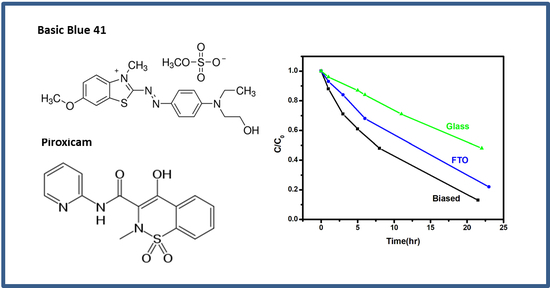
2.1. Photocatalytic and Photoelectrocatalytic Degradation of BB41
2.2. Photocatalytic and Photoelectrocatalytic Degradation of Piroxicam
2.3. Identification of Degradation Products of Piroxicam and Tentative Decomposition Pathways
3. Materials and Methods
3.1. Materials
3.2. Deposition of the Titania Nanoparticulate Film
3.3. Description of the Reactor
3.4. Measurements and Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bessegato, G.G.; Guaraldo, T.T.; de Brito, J.F.; Brugnera, M.F.; Zanoni, M.V.B. Achievements and Trends in Photoelectrocatalysis: From Environmental to Energy Applications. Electrocatalysis 2015, 6, 415–441. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Huang, J.Y.; Li, S.H.; Zang, S.N.; Deng, S.; Li, Q.S.; Zhang, K.Q.; Lai, Y.K. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: A review. Nanotechnol. Rev. 2016, 5, 75–112. [Google Scholar] [CrossRef]
- Tantis, I.; Stathatos, E.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic degradation of potential water pollutants in the presence of NaCl using nanocrystalline titania films. J. Chem. Technol. Biotechnol. 2015, 90, 1338–1344. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Q.; Liu, Z.; Zhao, Q.; Tian, X.; Li, H.; Gao, S. Preparation of 3D TiO2 nanotube arrays photoelectrode on Ti mesh for photoelectric conversion and photoelectrocatalytic removal of pollutant. Sep. Purif. Technol. 2018, 207, 206–212. [Google Scholar] [CrossRef]
- Paul, K.K.; Sreekanth, N.; Biroju, R.K.; Narayanan, T.N.; Giri, P.K. Solar light driven photoelectrocatalytic hydrogen evolution and dye degradation by metal-free few-layer MoS2 nanoflower/TiO2(B) nanobelts heterostructure. Sol. Energy Mater. Sol. Cells 2018, 185, 364–374. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, M.; Liu, L.; Wang, W.; Jiao, Y.; Zhou, Q. A novel photoelectrocatalytic system for organic contaminant degradation on a TiO2 nanotube (TNT)/Ti electrode. Electrochim. Acta 2010, 55, 5091–5099. [Google Scholar] [CrossRef]
- Turolla, A.; Bestetti, M.; Antonelli, M. Optimization of heterogeneous photoelectrocatalysis on nanotubular TiO2 electrodes: Reactor configuration and kinetic modeling. Chem. Eng. Sci. 2018, 182, 171–179. [Google Scholar] [CrossRef]
- Strataki, N.; Bekiari, V.; Stathatos, E.; Lianos, P. Effect of aggregation of dyes adsorbed on nanocrystalline titania films on the efficiency of photodegradation. J. Photochem. Photobiol. A Chem. 2007, 191, 13–18. [Google Scholar] [CrossRef]
- Tantis, I.; Bousiakou, L.; Frontistis, Z.; Mantzavinos, D.; Konstantinou, I.; Antonopoulou, M.; Katrikase, G.A.; Lianos, P. Photocatalytic and photoelectrocatalytic degradation of the drugomeprazole on nanocrystalline titania films in alkaline media: Effect of applied electrical bias on degradation and transformation products. J. Hazard. Mater. 2015, 294, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Peleyeju, M.G.; Umukoro, E.H.; Tshwenya, L.; Moutloali, R.; Babalola, J.O.; Arotiba, O.A. Photoelectrocatalytic water treatment systems: Degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2–exfoliated graphite electrode. RSC Adv. 2017, 7, 40571–40580. [Google Scholar] [CrossRef]
- Ghasemian, S.; Nasuhoglu, D.; Omanovic, S.; Yargeau, V. Photoelectrocatalytic degradation of pharmaceutical carbamazepine using Sb-doped Sn80%-W20%-oxide electrodes. Sep. Purif. Technol. 2017, 188, 52–59. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-driven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Mahajan, S.; Sharma, R.; Stathatos, E. Novel development of nanocrystalline kesterite Cu2ZnSnS4 thin film with high photocatalytic activity under visible light illumination. J. Phys. Chem. Solids 2018, 112, 37–42. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Asli, M.A.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Bouras, P.; Lianos, P. Photodegradation of dyes in aqueous solutions catalyzed by highly efficient nanocrystalline Titania films. J. Appl. Electrochem. 2005, 35, 831–836. [Google Scholar] [CrossRef]
- Lianou, A.; Frontistis, Z.; Chatzisymeon, E.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sonochemical oxidation of piroxicam drug: Effect of key operating parameters and degradation pathways. J. Chem. Technol. Biotechnol. 2018, 93, 28–34. [Google Scholar] [CrossRef]
- Bacsi, I.; Beres, V.B.; Kokai, Z.; Gonda, S.; Novak, Z.; Nagy, S.A.; Vasas, G. Effects of non-steroidal anti-inflammatory drugs on cyanobacteria and algae in laboratory strains and in natural algal assemblages. Environ. Pollut. 2016, 212, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshi, R.; Oku, T.; Suzuki, A.; Kikuchi, K.; Kikuchi, S.; Jeyadevan, B.; Cuya, J. Fabrication and Characterization of Copper System Compound Semiconductor Solar Cells. Adv. Mater. Sci. Eng. 2010, 2010, 562842. [Google Scholar] [CrossRef]
- Pop, L.C.; Drakopoulos, V.; Lianos, P. Photoelectrocatalytic hydrogen production using nanoparticulatetitania and a novel Pt/carbon electrocatalyst: The concept of the “Photoelectrocatalytic Leaf”. Appl. Surf. Sci. 2015, 333, 147–151. [Google Scholar] [CrossRef]
- Feng, L. Advanced Oxidation Processes for the Removal of Residual Non-Steroidal Anti-Inflammatory Pharmaceuticals from Aqueous Systems. Ph.D. Thesis, Earth Sciences, Université Paris-Est, Marne-la-Vallée, France, 2013. (In English). [Google Scholar]
- Doukas, E.; Balta, P.; Raptis, D.; Avgouropoulos, G.; Lianos, P. A Realistic Approach for Photoelectrochemical Hydrogen Production. Materials 2018, 11, 1269. [Google Scholar] [CrossRef] [PubMed]






| DP Code | Rt (Min) | Deprotonated Molecular Formula | m/z [Μ-H]− | Δ (ppm) | RDBE | MS2 m/z [Μ-H]− | Deprotonated Molecular Formula | Δ (ppm) | RDBE + |
|---|---|---|---|---|---|---|---|---|---|
| DP1 | 3.18 | C7H8NO2S | 170.0282 | 0.338 | 4.5 | 106.067 | C7H8N | 7.706 | 4.5 |
| DP2 | 4.76 | C8H6NO4S | 212.0021 | −1.14 | 6.5 | ||||
| DP3 | 6.85 | C8H6NO4S | 212.0019 | −1.800 | 6.5 | ||||
| C8H6NO2 | 148.0407 | 1.947 | 6.5 | ||||||
| DP4 | 7.25 | C8H6NO4S | 212.0017 | −2.744 | 6.5 | ||||
| DP5 | 8.12 | C15H12N3O5S | 346.0493 | 3.0 | 11.5 | 282.0872 | C15H12N3O3 | −4.235 | 11.5 |
| C15H12N3O3 | 282.0872 | −4.235 | 11.5 | 253.0280 | C10H9O4N2S | −3.441 | 7.5 | ||
| C10H9N2O4S | 253.0282 | −2.493 | 7.5 | ||||||
| DP6 | 8.29 | C15H12N3O5S | 346.0491 | −3.596 | 11.5 | 282.0876 | C15H12N3O3 | −2.817 | 11.5 |
| C15H12N3O3 | 282.0875 | −6.504 | 11.5 | 251.996 | C10H6O5NS | −2.565 | 8.5 | ||
| C10H6O5NS | 251.9963 | −3.596 | 8.5 | 226.0171 | C9H8NO4S | −3.813 | 6.5 | ||
| 182.9757 | C7H3O4S | −0.506 | 6.5 | ||||||
| PRX | 8.56 | C15H12N3O4S | 330.0547 | −2.242 | 11.5 | 266.0932 | C15H12N3O2 | −1.127 | 11.5 |
| C15H12N3O2 | 266.0930 | −1.916 | 11.5 | 210.0227 | C9H8O3NS | −1.796 | 6.5 | ||
| C9H8NO3S | 210.0224 | −2.796 | 6.5 | 169.9966 | C7H5O3S | 0.544 | 5.5 | ||
| C9H8NO | 146.0615 | 2.347 | 6.5 | ||||||
| DP7 | 9.42 | C16H14N3O4S | 344.0703 | −2.063 | 11.5 | 160.0771 | C10H10NO | 2.266 | 6.5 |
| C16H12N3O2 | 280.1086 | −2.035 | 11.5 | 280.1087 | C16H14O2N3 | −1.607 | 11.5 | ||
| C10H10NO3S | 224.0383 | −1.862 | 6.5 | 224.0384 | C10H10NO3S | −1.103 | 6.5 | ||
| C10H10NO | 160.0771 | 2.266 | 6.5 | 169.9968 | C7H5O3S | 2.083 | 5.5 | ||
| DP8 | 9.91 | C15H12N3O4S | 346.0495 | −2.238 | 11.5 | ||||
| C9H8N3O2 | 226.0174 | −2.485 | 6.5 | ||||||
| C9H8NO2 | 162.0565 | 2.765 | 6.5 | ||||||
| C8H5NO2 | 147.0328 | 1.654 | 7.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagiannis, I.; Koutsikou, G.; Frontistis, Z.; Konstantinou, I.; Avgouropoulos, G.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants. Catalysts 2018, 8, 455. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8100455
Papagiannis I, Koutsikou G, Frontistis Z, Konstantinou I, Avgouropoulos G, Mantzavinos D, Lianos P. Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants. Catalysts. 2018; 8(10):455. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8100455
Chicago/Turabian StylePapagiannis, Ioannis, Georgia Koutsikou, Zacharias Frontistis, Ioannis Konstantinou, George Avgouropoulos, Dionissios Mantzavinos, and Panagiotis Lianos. 2018. "Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants" Catalysts 8, no. 10: 455. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8100455