Effect of Chlorine-Containing VOCs on Silver Migration and Sintering in ZSM-5 Used in a TSA Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Deactivation Mechanisms of Ag@ZSM-5 Zeolite
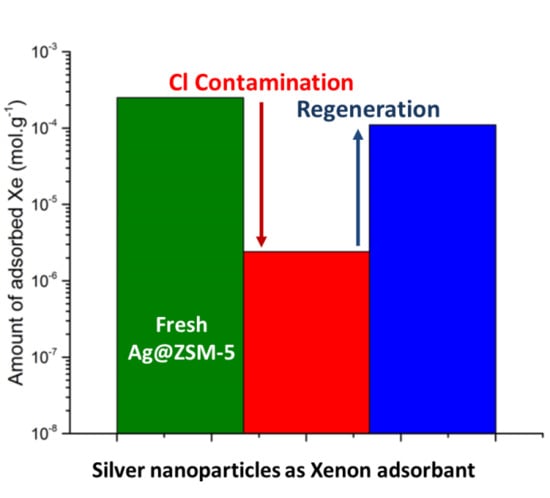
2.1.1. Loss of Adsorption Capacities
2.1.2. Identification of the AgCl Phase upon the Adsorption Step
2.1.3. Simulation of AgCl Nanoparticle Adsorption
2.2. Identification of the Chlorine-Containing VOCs
- Tenax T1 was placed directly behind the nitrogen membranes to identify the compounds in the gas flow.
- Tenax tube T2 was placed after a column containing 3 g of Ag@ZSM-5.
- Tenax tube T3 was placed after a column containing 3 g of Ag@ZSM-5, which was then eluted at 623 K with a nitrogen flow, in order to determine if the compounds are only physisorbed or if they react with the silver particles.
2.3. Regeneration Temperature of Ag@ZSM-5
2.3.1. Heat Treatment Effect
2.3.2. Study of the Regeneration of Ag@ZSM-5 at 623 K
2.4. Validation of Regeneration Process under TSA Cycles
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Adsorbent Synthesis
3.1.2. Degradation and Regeneration Steps
- The “fresh sample” corresponds to the Ag@ZSM-5 zeolite after heat treatment at 673 K in N2 as reported above.
- The “degraded sample” corresponds to Ag@ZSM-5 exposed to Cl-containing VOCs. The degradation protocol consists in feeding a column of fresh zeolite with a Cl-VOC concentrated stream. In order to concentrate the airstream with relevant VOCs encountered in the SPALAX system, atmospheric air was treated by six nitrogen generator membranes (UBE N2 Separator, UBE©, Tokyo, Japan) [25]. The degradation protocol was applied for a column filled with 13 g of fresh Ag@ZSM-5 at room temperature under a flow of air treated by membranes at 300 NL.h−1 for 168 h.
- The “regenerated sample” corresponds to the degraded sample mentioned above, which is then regenerated in a column by means of heat treatment in N2. The regeneration gas was nitrogen (BIP grade, Air Products©, Allentown, PA, USA) under a flow of 200 NL·h−1 for 48 h. After a study of the temperature effect (detailed below), the temperature chosen for the validation using a cyclic TSA process was 573 K.
3.1.3. Heat Treatments
3.2. Experimental Techniques
3.2.1. Isothermal Adsorption Experiments
3.2.2. XRD Analyses
3.2.3. XPS Analyses
3.2.4. Elemental Analyses
3.2.5. STEM Analyses
3.3. Bench Test for Aging of Ag@ZSM-5
3.4. Molecular Simulation
3.4.1. Preparation of AgCl Nanoparticles for Simulation
3.4.2. Grand Canonical Monte Carlo Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dubasov, Y.V.; Popov, Y.S.; Prelovskii, V.V.; Donets, A.Y.; Kazarinov, N.M.; Mishurinskii, V.V.; Popov, V.Y.; Rykov, Y.M.; Skirda, N.V. The АРИКС-01 Automatic Facility for Measuring Concentrations of Radioactive Xenon Isotopes in the Atmosphere. Instrum. Exp. Tech. 2005, 48, 373–379. [Google Scholar] [CrossRef]
- Cullen, S.C.; Gross, E.G. The Anesthetic Properties of Xenon in Animals and Human Beings, with Additional Observations on Krypton. Science 1951, 113, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Fraissard, J.; Ito, T. 129Xe n.m.r. study of adsorbed xenon: A new method for studying zeolites and metal-zeolites. Zeolites 1988, 8, 350–361. [Google Scholar] [CrossRef]
- Le Petit, G.; Douysset, G.; Ducros, G.; Gross, P.; Achim, P.; Monfort, M.; Raymond, P.; Pontillon, Y.; Jutier, C.; Blanchard, X.; et al. Analysis of Radionuclide Releases from the Fukushima Dai-Ichi Nuclear Power Plant Accident Part I. Pure Appl. Geophys. 2014, 171, 629–644. [Google Scholar] [CrossRef]
- Achim, P.; Monfort, M.; Le Petit, G.; Gross, P.; Douysset, G.; Taffary, T.; Blanchard, X.; Moulin, C. Analysis of Radionuclide Releases from the Fukushima Dai-ichi Nuclear Power Plant Accident Part II. Pure Appl. Geophys. 2014, 171, 645–667. [Google Scholar] [CrossRef]
- Topin, S.; Greau, C.; Deliere, L.; Hovesepian, A.; Taffary, T.; Le Petit, G.; Douysset, G.; Moulin, C. SPALAX new generation: New process design for a more efficient xenon production system for the CTBT noble gas network. J. Environ. Radioact. 2015, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Le Petit, G.; Cagniant, A.; Gross, P.; Douysset, G.; Topin, S.; Fontaine, J.P.; Taffary, T.; Moulin, C. SpalaxTM new generation: A sensitive and selective noble gas system for nuclear explosion monitoring. Appl. Radiat. Isot. 2015, 103, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.G. Zeolite Membranes for the Separation of Krypton and Xenon from Spent Nuclear Fuel Reprocessing Off-Gas. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2013. [Google Scholar]
- Häussinger, P.; Glatthaar, R.; Rhode, W.; Kick, H.; Benkmann, C.; Weber, J.; Wunschel, H.-J.; Stenke, V.; Leicht, E.; Stenger, H. Noble Gases. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Kerry, F.G. Industrial Gas Handbook: Gas Separation and Purification; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-0826-5. [Google Scholar]
- Fontaine, J.P.; Pointurier, F.; Blanchard, X.; Taffary, T. Atmospheric xenon radioactive isotope monitoring. J. Environ. Radioact. 2004, 72, 129–135. [Google Scholar] [CrossRef]
- Munakata, K.; Kanjo, S.; Yamatsuki, S.; Koga, A.; Ianovski, D. Adsorption of Noble Gases on Silver-mordenite. J. Nucl. Sci. Technol. 2003, 40, 695–697. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Ansón, A.; Koenig, A.; Kuznicki, T.M.; Haastrup, T.; Eyring, E.M.; Hunter, D. Xenon Adsorption on Modified ETS-10. J. Phys. Chem. C 2007, 111, 1560–1562. [Google Scholar] [CrossRef]
- Daniel, C.; Elbaraoui, A.; Aguado, S.; Springuel-Huet, M.A.; Nossov, A.; Fontaine, J.P.; Topin, S.; Taffary, T.; Deliere, L.; Schuurman, Y.; et al. Xenon Capture on Silver-Loaded Zeolites: Characterization of Very Strong Adsorption Sites. J. Phys. Chem. C 2013, 117, 15122–15129. [Google Scholar] [CrossRef]
- Deliere, L.; Topin, S.; Coasne, B.; Fontaine, J.P.; De Vito, S.; Den Auwer, C.; Solari, P.L.; Daniel, C.; Schuurman, Y.; Farrusseng, D. Role of Silver Nanoparticles in Enhanced Xenon Adsorption Using Silver-Loaded Zeolites. J. Phys. Chem. C 2014, 118, 25032–25040. [Google Scholar] [CrossRef]
- Monpezat, A.; Topin, S.; Deliere, L.; Farrusseng, D.; Coasne, B. Evaluation Methods of Adsorbents for Air Purification and Gas Separation at Low Concentration: Case Studies on Xenon and Krypton. Ind. Eng. Chem. Res. 2019, 58, 4560–4571. [Google Scholar] [CrossRef]
- Munakata, K.; Fukumatsu, T.; Yamatsuki, S.; Tanaka, K.; Nishikawa, M. Adsorption Equilibria of Krypton, Xenon, Nitrogen and Their Mixtures on Molecular Sieve 5A and Activated Charcoal. J. Nucl. Sci. Technol. 1999, 36, 818–829. [Google Scholar] [CrossRef]
- El-Roz, M.; Telegeiev, I.; Mordvinova, N.E.; Lebedev, O.I.; Barrier, N.; Behilil, A.; Zaarour, M.; Lakiss, L.; Valtchev, V. Uniform Generation of Sub-Nanometer Silver Clusters in Zeolite Cages Exhibiting High Photocatalytic Activity under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 28702–28708. [Google Scholar] [CrossRef]
- Severance, M.; Dutta, P.K. Evolution of Silver Nanoparticles within an Aqueous Dispersion of Nanosized Zeolite Y: Mechanism and Applications. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/abs/10.1021/jp5074957 (accessed on 23 April 2019).
- Deliere, L. Adsorption et Séparation des Gaz Rares sur des Adsorbants Dopés à L’argent. Ph.D. Thesis, Université Claude Bernard–Lyon, Lyon, France, 2015. [Google Scholar]
- Johnstone, A.H. CRC Handbook of Chemistry and Physics, 69th ed.; Chief, R.C., Weast, C.R.C., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1988; p. 2400. ISBN 0-8493-0369-5. J. Chem. Technol. Biotechnol. 1991, 50, 294–295. [Google Scholar] [CrossRef]
- Tammann, G. Lehrbuch der Metallkunde, 4th ed.; Verlag Voss: Berlin, Germany, 1929. [Google Scholar]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening. Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Koros, W.J.; Paul, D.R. Current Aspects of Membranes-Based Separation of Gases; Chenoweth, M.B., Ed.; Hardwood Academic Publishers: New York, NY, USA, 1984; Volume 5. [Google Scholar]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Von Der Ges. der Wiss. Zu Göttingen Math. Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Pellenq, R.J.M.; Nicholson, D. Grand Ensemble Monte Carlo Simulation of Simple Molecules Adsorbed in Silicalite-1 Zeolite. Langmuir 1995, 11, 1626–1635. [Google Scholar] [CrossRef]
- Zarzycki, P.; Kerisit, S.; Rosso, K.M. Molecular Dynamics Study of the Electrical Double Layer at Silver Chloride−Electrolyte Interfaces. J. Phys. Chem. C 2010, 114, 8905–8916. [Google Scholar] [CrossRef]
- Cheng, L.; Yin, C.; Mehmood, F.; Liu, B.; Greeley, J.; Lee, S.; Lee, B.; Seifert, S.; Winans, R.E.; Teschner, D.; et al. Reaction Mechanism for Direct Propylene Epoxidation by Alumina-Supported Silver Aggregates: The Role of the Particle/Support Interface. ACS Catal. 2014, 4, 32–39. [Google Scholar] [CrossRef]
- Liu, Z.; Ihl Woo, S. Recent Advances in Catalytic DeNOX Science and Technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Ouyang, R.; Liu, J.-X.; Li, W.-X. Atomistic Theory of Ostwald Ripening and Disintegration of Supported Metal Particles under Reaction Conditions. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/abs/10.1021/ja3087054 (accessed on 11 March 2019).
- Bartholomew, C.H.; Farrauto, R.J. Catalyst Deactivation: Causes, Mechanisms, and Treatment. In Fundamentals of Industrial Catalytic Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 260–336. ISBN 978-0-471-73007-1. [Google Scholar]









| Sample | Cl |
|---|---|
| Fresh Ag@ZSM-5 | <5 ppmw * |
| Degraded Ag@ZSM-5 | 0.40 wt.% |
| Sample | Cl |
|---|---|
| Fresh Ag@ZSM-5 | <5 ppmw |
| Degraded Ag@ZSM-5 | 0.40 wt.% |
| Regenerated Ag@ZSM-5 | 62 ppmw |
| σ (A) | ε (K) | Ref | ||
|---|---|---|---|---|
| Ag+ | Ag+ | 2.186 | 54.34519 | LB |
| Cl− | Cl− | 3.934 | 419.3958 | LB |
| H2O (SPCE) | H2O (SPCE) | 3.166 | 78.17659 | [27] |
| Xe | Xe | 3.849 | 281 | [28] |
| Ag+ | H2O (SPCE) | 2.676 | 65.18068 | [29] |
| Cl− | H2O (SPCE) | 3.55 | 181.0716 | [29] |
| Ag+ | Xe | 3.0175 | 123.5759 | LB |
| Cl− | H2O (SPCE) | 3.8915 | 343.2932 | LB |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monpezat, A.; Couchaux, G.; Thomas, V.; Artheix, A.; Deliere, L.; Gréau, C.; Topin, S.; Coasne, B.; Roiban, L.; Cardenas, L.; et al. Effect of Chlorine-Containing VOCs on Silver Migration and Sintering in ZSM-5 Used in a TSA Process. Catalysts 2019, 9, 686. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080686
Monpezat A, Couchaux G, Thomas V, Artheix A, Deliere L, Gréau C, Topin S, Coasne B, Roiban L, Cardenas L, et al. Effect of Chlorine-Containing VOCs on Silver Migration and Sintering in ZSM-5 Used in a TSA Process. Catalysts. 2019; 9(8):686. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080686
Chicago/Turabian StyleMonpezat, Arnaud, Gabriel Couchaux, Vincent Thomas, Antoine Artheix, Ludovic Deliere, Claire Gréau, Sylvain Topin, Benoit Coasne, Lucian Roiban, Luis Cardenas, and et al. 2019. "Effect of Chlorine-Containing VOCs on Silver Migration and Sintering in ZSM-5 Used in a TSA Process" Catalysts 9, no. 8: 686. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080686