Reactions of Experimentally Known Closo-C2B8H10 with Bases. A Computational Study
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
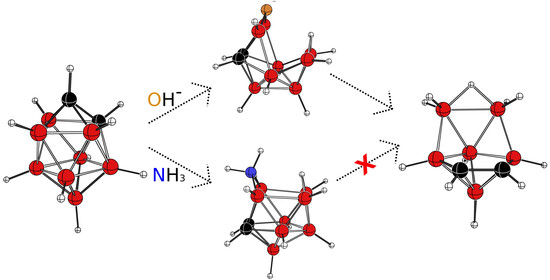
3.1. The Reaction with Hydroxides
3.2. The Reaction with Amines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hnyk, D.; Wann, D.A. Boron: The Fifth Element. In Challenges and Advances in Computational Chemistry and Physics; Hnyk, D., McKee, M., Eds.; Springer: Dordrecht, The Netherlands, 2016; Volume 20, pp. 17–48. [Google Scholar]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- McKee, M.L. Boron: The Fifth Element. In Challenges and Advances in Computational Chemistry and Physics; Hnyk, D., McKee, M., Eds.; Springer: Dordrecht, The Netherlands, 2016; Volume 20, pp. 121–138. [Google Scholar]
- McKay, D.; Macgregor, S.A.; Welch, A.J. Isomerisation of nido-[C2B10H12]2− Dianions: Unprecedented Rearrangements and New Structural Motifs in Carborane Cluster Chemistry. Chem. Sci. 2015, 6, 3117–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shameena, O.; Pathak, B.; Jemmis, E.D. Theoretical Study of the Reaction of B20H16 with MeCN: Closo/Closo to Closo/Nido Conversion. Inorg. Chem. 2008, 47, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Štíbr, B.; Holub, J.; Bakardjiev, M.; Lane, P.D.; McKee, M.L.; Wann, D.A.; Hnyk, D. Unusual Cage Rearrangements in 10-Vertex nido-5,6-Dicarbaborane Derivatives: An Interplay between Theory and Experiment. Inorg. Chem. 2017, 56, 852–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleyer, P.V.R.; Najafian, K. Stability and Three-Dimensional Aromaticity of closo-Monocarbaborane Anions, CBn−1Hn−, and closo-Dicarboranes, C2Bn−2Hn. Inorg. Chem. 1998, 37, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Bakardjiev, M.; Štíbr, B.; Holub, J.; Padělková, Z.; Růžička, A. Simple Synthesis, Halogenation, and Rearrangement of closo-1,6-C2B8H10. Organometallics 2015, 34, 450–454. [Google Scholar] [CrossRef]
- Tok, O.L.; Bakardjiev, M.; Štíbr, B.; Hnyk, D.; Holub, J.; Padělková, Z.; Růžička, A. Click Dehydrogenation of Carbon-Substituted nido-5,6-C2B8H12 Carboranes: A General Route to closo-1,2-C2B8H10 Derivatives. Inorg. Chem. 2016, 55, 8839–8843. [Google Scholar] [CrossRef] [PubMed]
- Gimarc, B.M.; Ott, J.J. Isomerization of Carboranes C2B6H18, C2B8H10 and C2B9H11 by the Diamond-Square-Diamond Rearrangement. J. Am. Chem. Soc. 1987, 109, 1388–1392. [Google Scholar] [CrossRef]
- Janoušek, Z.; Fusek, J.; Štíbr, B. The First Example of a Direct closo to arachno Cluster Expansion Reaction in Boron Cluster Chemistry. J. Chem. Soc. Dalton Trans. 1992, 2649–2650. [Google Scholar] [CrossRef]
- Hnyk, D.; Holub, J. Handles for the Dicarbadodecaborane Basket Based on [arachno-4,5-C2B8H13]−: Oxygen. Dalton Trans. 2006, 2620–2622. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.V.R.; Najafian, K.; Mebel, A.M. The Large closo-Borane Dianions, BnHn2− (n = 13–17) Are Aromatic, Why Are They Unknown? Inorg. Chem. 1998, 37, 6765–6772. [Google Scholar] [CrossRef] [PubMed]
- For Example, Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley and Sons: Chichester, Sussex, UK, 1976. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J. Phys. Chem. B 2009, 113, 4538–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Jelínek, T.; Štíbr, B.; Heřmánek, S.; Plešek, J. A New Eight-Vertex arachno-Dicarbaborane anion, [4,5-C2B6H11]−. J. Chem. Soc. Chem. Commun. 1989, 804–805. [Google Scholar] [CrossRef]
- Janoušek, Z.; Dostál, R.; Macháček, J.; Hnyk, D.; Štíbr, B. The First Member of the Eleven-Vertex Azadicarbaborane Series, 1,6,9-NC2B8H13 and its N-alkyl Derivatives. Dalton Trans. 2006, 4664–4671. [Google Scholar] [CrossRef] [PubMed]
- Plešek, J.; Štíbr, B.; Hnyk, D.; Jelínek, T.; Heřmánek, S.; Kennedy, J.D.; Hofmann, M.; Schleyer, P.V.R. Dicarbaheteroborane Chemistry. Representatives of Two Eleven-Vertex Dicarbaazaundecaborane Families: Nido-10,7,8-NC2B8H11, Its N-Substituted Derivatives, and arachno-1,8,11-NC2B8H13. Inorg. Chem. 1998, 37, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, A.; Goijens, G.; Nguyen, M.T. C2B7H9: Snapshots of a Rearranging Carborane. J. Am. Chem. Soc. 1994, 116, 9395–9396. [Google Scholar] [CrossRef]
- Lipscomb, W.N. Framework Rearrangement in Boranes and Carboranes. Science 1966, 153, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wade, K. Structural and Bonding Patterns in Cluster Chemistry. Adv. Inorg. Chem. Radiochem. 1976, 18, 1–66. [Google Scholar]









| Notation | ∆G (solv) 1 | ∆G (aq,298K) 2 | Notation | ∆G (solv) 1 | ∆G (aq,298K) 2 |
|---|---|---|---|---|---|
| C2B8H10 + OH (−) 3 | C2B8H10 + NH3 4 | ||||
| OH(−) | −94.76 | NH3 | −3.67 | ||
| H2O | −2.05 | H2BNH2 | −1.24 | ||
| H2BOH | −3.82 | oTS1-N | −4.70 | 32.1 | |
| OBOH | −14.65 | oA-N | −11.06 | 15.8 | |
| B(OH)3 | oTS2-N | −0.43 | 50.3 | ||
| oTS1-O | −53.74 | 27.2 | oP1-N | −3.75 | −1.3 |
| oA-O | −46.19 | 2.0 | oTS3-N | −0.64 | 43.8 |
| oTS2O | −42.58 | 10.0 | oB-N | −0.39 | 23.8 |
| oB-O | −44.80 | 1.8 | oTS4-N | −1.46 | 32.8 |
| oTS3-O | −39.89 | 14.5 | oC-N | −0.34 | 24.4 |
| oP1-O | −40.16 | −18.4 | oTS5-N | −0.01 | 32.3 |
| 4,5-C2B6H11(−) | −39.67 | −50.7 | oD-N | 0.27 | 31.2 |
| oC-O | −41.59 | −18.2 | oTS6-N | 0.81 | 39.5 |
| oTS4-O | −45.21 | 18.5 | P2-N | −3.48 | 7.3 |
| oD-O | −47.70 | −2.2 | oTS7-N | −1.01 | 38.3 |
| oTS5-O | −48.36 | 9.1 | oE-N | −0.55 | 29.5 |
| oE-O | −47.59 | 0.0 | oTS8-N | 0.49 | 36.4 |
| oTS6-O | −47.61 | 0.2 | oP3-N | −3.20 | 11.1 |
| oF-O | −45.17 | −1.2 | oTS9-N | −3.06 | 32.1 |
| oTS7-O | −43.15 | 8.8 | oF-N | −3.30 | 29.7 |
| oP2-O | −45.07 | −18.8 | oTS10-N | −6.51 | 29.8 |
| oTS8-O | −58.47 | 6.2 | oG-N | −2.08 | 25.1 |
| oG-O | −63.94 | 1.6 | oTS11-N | −2.74 | 35.0 |
| oG′-O | −63.85 | 1.6 | oH-N | −2.77 | 26.5 |
| oTS9-O | −50.19 | 7.9 | oTS12-N | −2.22 | 39.1 |
| oH-O | −53.32 | 4.6 | oI-N | −1.85 | 32.0 |
| oTS10-O | −51.94 | 5.7 | oTS13-N | −1.97 | 32.8 |
| oI-O | −66.84 | 0.1 | oP4-N | −2.27 | −5.5 |
| oJ-O | −60.59 | −1.1 | |||
| oTS11-O | −58.96 | −1.5 | |||
| oK-O | −48.64 | −27.9 | |||
| oK′-O | −44.34 | −26.8 | |||
| oTS12-O | −45.37 | -19.5 | |||
| oL-O | −51.68 | −26.9 | |||
| oM-O | −56.47 | −28.4 | |||
| oM′-O | −53.96 | −29.2 | |||
| oTS13-O | −58.13 | −4.2 | |||
| 4,5-C2B6H11(−) | −39.43 | −41.7 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holub, J.; Fanfrlík, J.; McKee, M.L.; Hnyk, D. Reactions of Experimentally Known Closo-C2B8H10 with Bases. A Computational Study. Crystals 2020, 10, 896. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100896
Holub J, Fanfrlík J, McKee ML, Hnyk D. Reactions of Experimentally Known Closo-C2B8H10 with Bases. A Computational Study. Crystals. 2020; 10(10):896. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100896
Chicago/Turabian StyleHolub, Josef, Jindřich Fanfrlík, Michael L. McKee, and Drahomír Hnyk. 2020. "Reactions of Experimentally Known Closo-C2B8H10 with Bases. A Computational Study" Crystals 10, no. 10: 896. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10100896