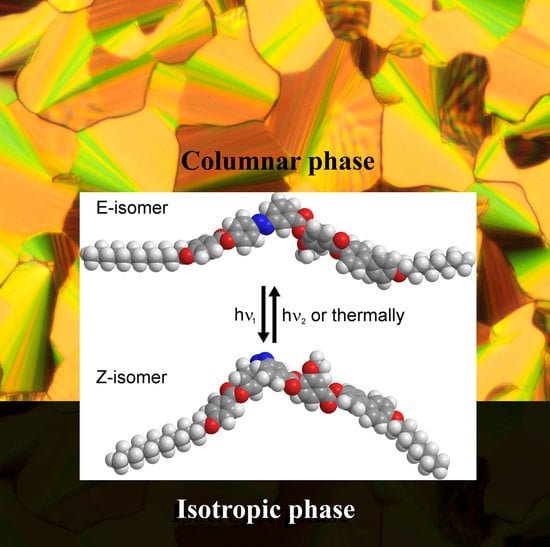
Photosensitive Bent-Core Compounds with Azo-Group Attached to the Central Ring
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Methods and Equipment
3. Results and Discussion
3.1. Mesomorphic Properties
3.2. X-ray Measurements
3.3. Photosensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bisoyi, H.K.; Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-Driven Control of the Helical Axis of Self-Organized Soft Helical Superstructures. Adv. Mater. 2018, 30, e1706512. [Google Scholar] [CrossRef] [PubMed]
- Niori, T.; Sekine, T.; Watanabe, J.; Furukawa, T.; Takezoe, H. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 1996, 6, 1231–1233. [Google Scholar] [CrossRef]
- Link, D.R.; Natale, G.; Shao, R.; MacLennan, J.E.; Clark, N.A.; Körblova, E.; Walba, D.M. Spontaneous Formation of Macroscopic Chiral Domains in a Fluid Smectic Phase of Achiral Molecules. Science 1997, 278, 1924–1927. [Google Scholar] [CrossRef]
- Takezoe, H.; Takanishi, Y. Bent-Core Liquid Crystals: Their Mysterious and Attractive World. Jpn. J. Appl. Phys. 2006, 45, 597–625. [Google Scholar] [CrossRef]
- Gimeno, N.; Ros, M.B. Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 4, pp. 603–679. [Google Scholar]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Weissflog, W.; Murthy, H.S.; Diele, S.; Pelzl, G. Relationships between molecular structure and physical properties in bent-core mesogens. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 2657–2679. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-core liquid crystals: Polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 16, 907–961. [Google Scholar] [CrossRef]
- Kohout, M.; Svoboda, J.; Novotná, V.; Glogarová, M.; Pociecha, D. Non-symmetrical bent-shaped liquid crystals with five ester groups. Liq. Cryst. 2010, 37, 987–996. [Google Scholar] [CrossRef]
- Kohout, M.; Kozmík, V.; Slabochová, M.; Tůma, J.; Svoboda, J.; Novotná, V.; Pociecha, D. Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core. Liq. Cryst. 2014, 42, 87–103. [Google Scholar] [CrossRef]
- Novotná, V.; Hamplová, V.; Kašpar, M.; Glogarová, M.; Pociecha, D. Switching of transformation from racemic to homochiral state in new liquid crystalline monomers with bent-core molecule. Liq. Cryst. 2005, 32, 1115–1123. [Google Scholar] [CrossRef]
- Szydlowska, J.; Mieczkowski, J.; Matraszek, J.; Bruce, D.W.; Gorecka, E.; Pociecha, D.; Guillon, D. Bent-core liquid crystals forming two- and three-dimensional modulated structures. Phys. Rev. E 2003, 67, 031702. [Google Scholar] [CrossRef]
- Gorecka, E.; Vaupotič, N.; Pociecha, D.; Čepič, M.; Mieczkowski, J. Switching Mechanism in Polar Columnar Mesophases Made of Bent-Core Molecules. Chem. Phys. Chem. 2005, 6, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaasar, M.; Prehm, M.; Tschierske, C. Influence of halogen substituent on the mesomorphic properties of five-ring banana-shaped molecules with azobenzene wings. Liq. Cryst. 2013, 40, 656–668. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Nagaraj, M.; Vij, J.K.; Tschierske, C. A Liquid Crystalline Phase with Uniform Tilt, Local Polar Order and Capability of Symmetry Breaking. Adv. Mater. 2013, 25, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M.; Prehm, M.; Tschierske, C. Helical Nano-crystallite (HNC) Phases: Chirality Synchronization of Achiral Bent-Core Mesogens in a New Type of Dark Conglomerates. Chem. A Eur. J. 2016, 22, 6583–6597. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; May, K.; Eremin, A.; Tschierske, C. 4-Cyanoresorcinol-Based Bent-Core Mesogens with Azobenzene Wings: Emergence of Sterically Stabilized Polar Order in Liquid Crystalline Phases. Adv. Funct. Mater. 2013, 24, 1703–1717. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Tschierske, C. New azobenzene containing bent-core liquid crystals based on disubstituted resorcinol. Liq. Cryst. 2013, 41, 126–136. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Tschierske, C. A new room temperature dark conglomerate mesophase formed by bent-core molecules combining 4-iodoresorcinol with azobenzene units. Chem. Commun. 2013, 49, 11062–11064. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M. Azobenzene-containing bent-core liquid crystals: An overview. Liq. Cryst. 2016, 43, 2208–2243. [Google Scholar] [CrossRef]
- Jing, H.; Xu, M.; Xiang, Y.; Wang, E.; Liu, D.; Poryvai, A.; Kohout, M.; Éber, N.; Buka, Á. Light Tunable Gratings Based on Flexoelectric Effect in Photoresponsive Bent-Core Nematics. Adv. Opt. Mater. 2019, 7, 1801790. [Google Scholar] [CrossRef]
- Nagaveni, N.; Raghuvanshi, P.; Roy, A.; Prasad, V. Azo-functionalised achiral bent-core liquid crystalline materials: Effect of presence of –N=N– linkage at different locations in the molecular architecture. Liq. Cryst. 2013, 40, 1238–1254. [Google Scholar] [CrossRef]
- Nagaveni, N.G.; Roy, A.; Prasad, V. Achiral bent-core azo compounds: Effect of different types of linkage groups and their direction of linking on liquid crystalline properties. J. Mater. Chem. 2012, 22, 8948–8959. [Google Scholar] [CrossRef]
- Srinivasan, M.V.; Kannan, P.; Roy, A. Investigations on photo and electrically switchable asymmetric bent-core liquid crystals. J. Mater. Sci. 2012, 48, 2433–2446. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Brautzsch, M.; Tschierske, C. Dark conglomerate phases of azobenzene derived bent-core mesogens—Relationships between the molecular structure and mirror symmetry breaking in soft matter. Soft Matter 2014, 10, 7285–7296. [Google Scholar] [CrossRef] [Green Version]
- Alaasar, M.; Prehm, M.; Brautzsch, M.; Tschierske, C. 4-Methylresorcinol based bent-core liquid crystals with azobenzene wings—A new class of compounds with dark conglomerate phases. J. Mater. Chem. C 2014, 2, 5487–5501. [Google Scholar] [CrossRef] [Green Version]
- Šmahel, M.; Poryvai, A.; Xiang, Y.; Pociecha, D.; Troha, T.; Novotná, V.; Svoboda, J.; Kohout, M. Photosensitive bent-core nematic liquid crystals with various linking units in the side arms: Structure-properties relationships. J. Mol. Liq. 2020, 306, 112743. [Google Scholar] [CrossRef]
- Nagaveni, N.G.; Prasad, V.; Roy, A. Azo functionalised achiral bent-core liquid crystals: Observation of photo-induced effects in B7 and B2 mesophases. Liq. Cryst. 2013, 40, 1405–1416. [Google Scholar] [CrossRef]
- Lazarev, G.; Chen, P.-J.; Strauss, J.; Fontaine, N.; Forbes, A. Beyond the display: Phase-only liquid crystal on Silicon devices and their applications in photonics. Opt. Express 2019, 27, 16206–16249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hsiang, E.-L.; Deng, M.-Y.; Wu, S.-T. Mini-LED, Micro-LED and OLED displays: Present status and future perspectives. Light. Sci. Appl. 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Monika, M.; Prasad, V.; Nagaveni, N. Hockey stick-shaped azo compounds: Effect of linkage groups and their direction of linking on mesomorphic properties. Liq. Cryst. 2015, 42, 1490–1505. [Google Scholar] [CrossRef]
- Prasad, V.; Gowdru, N.N.; Manjunath, M. Thermally stable azo-substituted bent-core nematogens: Observation of chiral domains in the nematic mesophases composed of smectic nano clusters. Liq. Cryst. 2017, 45, 666–679. [Google Scholar] [CrossRef]
- Kašpar, M.; Hamplová, V.; Novotná, V.; Glogarová, M.; Vaněk, P. New banana-type liquid crystal with a methoxy group substituted near the central ring. J. Mater. Chem. 2002, 12, 2221–2224. [Google Scholar] [CrossRef]
- Novotná, V.; Hamplová, V.; Kaspar, M.; Glogarová, M. New chlorine-substituted ferroelectric liquid crystals with four aromatic rings in the mesogenic core. Liq. Cryst. 2002, 29, 1435–1439. [Google Scholar] [CrossRef]
- Cigl, M.; Bubnov, A.; Kašpar, M.; Hampl, F.; Hamplová, V.; Pacherová, O.; Svoboda, J. Photosensitive chiral self-assembling materials: Significant effects of small lateral substituents. J. Mater. Chem. C 2016, 4, 5326–5333. [Google Scholar] [CrossRef]
- Rau, H.; Dürr, H.; Bouas-Laurent, H. (Eds.) Photochromism Molecules and Systems; Chapter 4: Azo Compounds; Elsevier B.V.: Amsterdam, The Netherlands, 2003; pp. 165–192. [Google Scholar]











| Sample | m.p./°C (H/kJmol) | Tcr/°C (H/kJmol) | M | Tiso/°C (H/kJmol) | Iso |
|---|---|---|---|---|---|
| 8BVJH12 | 125 (+29.3) | 103 (−26.0) | B1 | 150 (−18.2) | • |
| LJV10/10 | 58 (+45.1) | 43 (−36.6) | – | • | |
| LJ10/10 | 105 (+26.7) | 67 (−9.9) | B1Rev | 115 (−15.6) | • |
| T (°C) | a (Å) | b (Å) |
|---|---|---|
| 145 | 65.8 | 50.3 |
| 130 | 62.8 | 50.6 |
| 115 | 58.9 | 51.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cigl, M.; Hamplová, V.; Pociecha, D.; Novotná, V. Photosensitive Bent-Core Compounds with Azo-Group Attached to the Central Ring. Crystals 2020, 10, 1030. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111030
Cigl M, Hamplová V, Pociecha D, Novotná V. Photosensitive Bent-Core Compounds with Azo-Group Attached to the Central Ring. Crystals. 2020; 10(11):1030. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111030
Chicago/Turabian StyleCigl, Martin, Věra Hamplová, Damian Pociecha, and Vladimíra Novotná. 2020. "Photosensitive Bent-Core Compounds with Azo-Group Attached to the Central Ring" Crystals 10, no. 11: 1030. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111030