Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2) 2(H2O)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Crystal Data and Structure Determination
2.3. Physicochemical Characterization
2.4. Simulation Details
2.4.1. Hirshfeld Surface Analysis
2.4.2. Computer Morphology Simulation
3. Results and Discussion
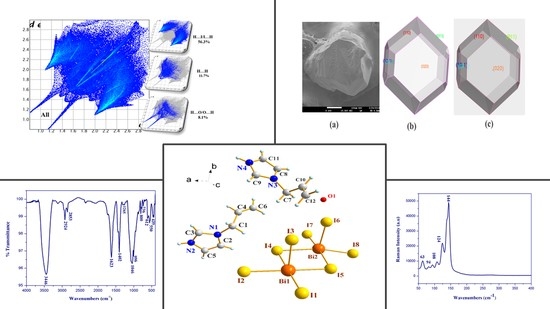
3.1. Energy-Dispersive X-ray Spectroscopy (SEM-EDX)
3.2. Structure Description
3.3. Hirshfeld Surface Analysis
3.4. Prediction of Crystal Morphology
3.5. Infrared (IR) and Raman Spectroscopy
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Ali, S.B.; Ferretti, V.; Bianco, L.D.; Spizzo, F.; Belhouchet, M. Structural, vibrational, optical properties and theoretical studies of a new organic inorganic material: Tris-acetoguanaminium hexachlorobismuthate monohydrate. J. Mol. Struct. 2020, 1199, 126986. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, X.; Yang, Y.; Yang, S.; Zhao, W.; Wumaier, T.; Wei, D.; Deng, W.; Han, K. Air-Stable, Lead-Free Zero-Dimensional Mixed Bismuth-Antimony Perovskite Single Crystals with Ultrabroad Band Emission. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, P.; Jakubas, R.; Piecha-Bisiorek, A.; Bator, G.; Chanski, M.; Medycki, W.; Wuttke, J. Organic-inorganic hybrid crystals, (2,4,6-CH3PyH)3Sb2Cl9 and (2,4,6-CH3PyH)3Bi2Cl9. Crystal structure characterization and tunneling of CH3 groups studied by 1H NMR and neutron spectroscopy. Polyhedron 2018, 139, 249–256. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, Z.K.; Liao, L.S. Progress of Lead-Free Halide Double Perovskites. Adv. Energy Mater. 2019, 1803150. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Neu, J.; Zhou, Y.; Chaaban, M.; Lee, S.; Worku, M.; Chen, B.; Clark, R.J.; Cheng, W.; et al. Green Emitting Single-Crystalline Bulk Assembly of Metal Halide Clusters with NearUnity Photoluminescence Quantum Efficiency. ACS Energy Lett. 2019, 4, 1579–1583. [Google Scholar] [CrossRef]
- Ahern, J.C.; Nicholas, A.D.; Kelly, A.W.; Chan, B.; Pike, R.D.; Patterson, H.H. A terbium chlorobismuthate(III) double salt: Synthesis, structure, and photophysical properties. Inorg. Chim. Acta 2018, 478, 71–76. [Google Scholar] [CrossRef]
- AKelly, W.; Nicholas, A.; Ahern, J.C.; Chan, B.; Patterson, H.H.; Pike, R.D. Alkali metal bismuth (III) chloride double salts. J. Alloy. Compd. 2016, 670, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Sorg, J.R.; Wehner, T.; Matthes, P.R.; Sure, R.; Grimme, S.; Heine, J.K. Müller- Buschbaum. Bismuth as a versatile cation for luminescence in coordination polymers from BiX3/4,4’-bipy: Understanding of photophysics by quantum chemical calculations and structural parallels to lanthanides. Dalton. Trans. 2018, 47, 7669–7681. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Dehnhardt, N.; Schmid, M.; Klein, B.P.; Ruppenthal, L.; Müller, P.; Zugermeier, M.; Gottfried, J.M.; Lippert, S.; Halbich, M.U.; et al. Color change effect in an organic-inorganic hybrid material based on a porphyrin diacid. J. Phys. Chem. C 2016, 120, 28363–28373. [Google Scholar] [CrossRef] [Green Version]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Troshin, P.A. Highly Efficient All-Inorganic Planar Heterojunction Perovskite Solar Cells Produced by Thermal Coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2017, 8, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.M.; Ardo, S. Hybrid organic-inorganic solar cells based on bismuth iodide and 1,6-hexanediammonium dication. J. Mater. Chem. A 2016, 4, 6837–6841. [Google Scholar] [CrossRef]
- Sichert, J.A.; Hemmerling, A.; Daw, C.C.; Urban, A.S.; Feldmann, J. Tuning the optical bandgap in layered hybrid perovskites through variation of alkyl chain length. APL Mater. 2019, 7, 041116. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond methylammonium lead iodide: Prospects for the emergent field of ns2 containing solar absorbers. Chem. Commun. 2017, 53, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polynuclear halide complexes of Bi(III): From structural diversity to the new properties. Coord. Chem. Rev. 2016, 312, 1–21. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with halopyridinium cations: Appearance or non-appearance of unusual colouring. Cryst. Eng. Commun. 2018, 20, 776. [Google Scholar] [CrossRef]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and Two-Dimensional Iodine-Rich Iodobismuthate(III) Complexes: Structure, Optical Properties, and Features of Halogen Bonding in the Solid State. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Bismuth(III) Halide Complexes: New Structural Types and New Application Areas. Russ. J. Inorg. Chem. 2017, 62, 1789–1796. [Google Scholar] [CrossRef]
- AUsoltsev, N.; Elshobaki, M.; Adonin, S.A.; Frolova, L.A.; Derzhavskaya, T.; Abramov, P.A.; Anokhin, D.V.; Korolkov, I.V.; Luchkin, S.Y.; Dremova, N.N.; et al. Polymeric iodobismuthates {[Bi3I10]} and {[BiI4]} with N-heterocyclic cations: Promising perovskite-like photoactive materials for electronic devices. J. Mater. Chem. A 2019, 7, 5957–5966. [Google Scholar] [CrossRef]
- Attia, S.; Chaari, N.; Chaabouni, S. Synthesis, Crystal Structure, and Dielectric Properties of (3-Aminopropyl-imidazolium) Pentachlorobismuthate(III) [C6H13N3]BiCl5. J. Cluster. Sci. 2015. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Yegorova, I.V.; Klepikov, N.N.; Boyarkina, E.A.; Sharutin, O.K. Synthesis and structure of bismuth complexes [Ph3MeP]6+[BiI3Br3]3-[Bi2I6Br3]3-.H2O2, [Ph3EtP]3+[Bi2I9]3-, n[Ph3MeP]3+[Bi3I12]3-, [Ph3(iso-Pr)P]3+[Bi3I12]3-.2Me2C=O, and [Ph4Bi]3+[Bi5I18]3-. Russ. J. Inorg. Chem. 2009, 54, 52. [Google Scholar] [CrossRef]
- Yelovik, N.A.; Shestimerov, T.A.; Bykov, M.A.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Synthesis, structure, and properties of LnBiI6.13H2O (Ln = La, Nd). Russ. Chem. Bull. 2017, 66, 1196. [Google Scholar] [CrossRef]
- Krautscheid, H. Synthese und Kristallstrukturen von (Ph4P)4[Bi8I28], (nBu4N)[Bi2I7] und (Et3PhN)2[Bi3I11]- Iodobismutate mit isolierten bzw polymeren Anionen. Z. Anorg. Allg. Chem. 1995, 621, 2049. [Google Scholar] [CrossRef]
- Buikin, P.A.; Rudenko, A.Y.; Baranchikov, A.E.; Ilyukhin, A.B.; Kotov, V.Y. 1D-Bromobismuthates of Dipyridinoalkane Derivatives. Russ. J. Coord. Chem. 2018, 44, 373–379. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From Isolated Anions to Polymer Structures through Linking with I2: Synthesis, Structure, and Properties of Two Complex Bismuth(III) Iodine Iodides. Inorg. Chem. 2018, 57, 4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Feng, Q.Y.; Wang, Q.L.; Huang, X.; Chen, D.; Zhou, J. A New Iodobismuthate-Based Hybrid Containing Mixed Iodobismuthate Clusters Templated by Diammonium Cation: Structure and Photocurrent Response. J. Cluster. Sci. 2018, 29, 367. [Google Scholar] [CrossRef]
- Bruker APEX3; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Bruker SAINT; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. SADABS Bruker; AXS Inc.: Madison, WI, USA, 2017. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer (Version 3.1); University of Western: Perth, Australia, 2012. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Materials Studio, Version 7.0; Accelrys Software Inc.: San Diego, CA, USA, 2013.
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Goforth, A.M.; Gardinie, J.R.; Smith, M.D.; Peterson, L.R.; Loye, H.C.Z. [Ru(2,2′-bipy)3]2[Bi4I 16]: A bimetallic inorganic-organic complex consisting of a d-metal coordination cation and a polynuclear iodobismuthate anion. Inorg. Chem. Commun. 2005, 8, 684–688. [Google Scholar] [CrossRef]
- Carmalt, C.J.; Farrugia, L.J.; Norman, N.C. Synthesis and X-ray Crystal Structure of a Polymeric Iodobismuthate Anion. Z. Naturforsch. B 1995, 50, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, R.; Ejsmont, K. Crystal structure of a novel bismuth phthalocyanine-bismuth iodide complex. J. Mol. Struct. 1999, 474, 275–281. [Google Scholar] [CrossRef]
- Bondi, A.J. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- GDestri, L.; Marrazzo, A.; Rescifina, A.; Punzo, F. Crystal Morphologies and Polymorphs in Tolbutamide Microcrystalline Powder. J. Pharm. Sci. 2013, 102, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.; Bennema, P. The attachment energy as a habit controlling factor: I. Theoretical considerations. J. Cryst. Growth. 1980, 49, 145–156. [Google Scholar] [CrossRef]
- Piecha, A.; Jakubas, R.; Pietraszko, A. Phase transitions and electric properties of imidazolium chlorobismuthate(III): [C3H5N2]6[Bi4Cl18]. J. Mol. Struct. 2007, 829, 149. [Google Scholar] [CrossRef]
- Belkyal, I.; Mokhlisse, R.; Tanouti, B.; Chanh, N.B.; Couzi, M. X-ray Diffraction and Raman Scattering in (CH3NH3)3Bi2Cl9 (MACB) Single Crystals. Phys. Stat. Sol. 1993, 136, 45. [Google Scholar] [CrossRef]
- Kuok, M.H.; Ng, S.C.; Iwata, M.; Ishibashi, Y. A Raman study of the phase transition in (CH3NH3)5Bi2Cl11. Solid State Commun. 1993, 86, 151. [Google Scholar] [CrossRef]
- Carpentier, P. Étude des transitions de phase du cristal ferroélectrique (CH3NH3)5BI2CL11 (PMACB): structure et dynamique Phase transitions study of the ferroelectric crystal (CH3NH3)5BI2CL11 (MAPCB). Thesis, Universite of Lille 1, Lille, France, 1995. Available online: http://ori-nuxeo.univ-lille1.fr/nuxeo/site/esupver (accessed on 15 May 2020).
- Hooper, M.A.; James, D.W. Vibrational spectra of some Group Vb halides. III. A far-infrared and Raman spectral study of some Hexahalogeno-, Pentahalogeno-, and Tetrahalogeno-bismuthate(III) and -antimonate(III) salts. Aust. J. Chem. 1973, 26, 1401. [Google Scholar] [CrossRef]











| Empirical Formula | Bi4I16·4(C6H9N2)·2(H2O) |
|---|---|
| Formula weight (g/mol) | 3338.96 |
| Crystal system, space group | Monoclinic, P21/n |
| a (Å) | 11.312 (6) |
| b (Å) | 25.985 (2) |
| c (Å) | 11.879 (7) |
| β (°) | 110.05 (2) |
| V (Å3) | 3280 (3) |
| Z | 2 |
| µ (mm−1) | 18.26 |
| Dx (Mg∙m−3) | 3.381 |
| F(000) | 3.381 |
| Crystal size (mm) | 0.34 × 0.33 × 0.20 |
| Crystal habit | Block, orange |
| θmin/θmax (°) | 2.5/28.4 |
| Measured reflections | 113,427 |
| Independent reflections | 8177 |
| Observed reflections with I > 2σ(I) | 6751 |
| Rint | 0.116 |
| Data/restraints/parameters | 8177/0/245 |
| R[F2 > 2σ(F2)] | 0.042 |
| wR(F2) | 0.091 |
| GooF = S | 1.086 |
| Δρmax/Δρmin (e Å−3) | 1.55/−1.39 |
| Bond Distances (Ả) | |||
| Bi1–I3 | 2.9033 (6) | Bi2–I6 | 2.8918 (7) |
| Bi1–I1 | 2.9219 (6) | Bi2–I7 | 2.9581 (6) |
| Bi1–I2 | 3.0228 (6) | Bi2–I5 | 3.2456 (6) |
| Bi1–I5 | 3.1416 (6) | Bi2–I2 i | 3.3271 (6) |
| Bi1–I4 | 3.3196 (6) | Bi2–I4 | 3.3418 (6) |
| Bi1–I4 i | 3.3309 (6) | I2–Bi2 i | 3.3272 (6) |
| Bi2–I8 | 2.8898 (7) | I4–Bi1 i | 3.3308 (6) |
| Bond Angles (°) | |||
| I3–Bi1–I1 | 92.74 (2) | I8–Bi2–I6 | 96.58 (3) |
| I3–Bi1–I2 | 89.82 (2) | I8–Bi2–I7 | 95.71 (2) |
| I1–Bi1–I2 | 94.571 (2) | I6–Bi2–I7 | 91.90 (2) |
| I3–Bi1–I5 | 93.38 (2) | I8–Bi2–I5 | 87.97 (2) |
| I1–Bi1–I5 | 90.259 (2) | I6–Bi2–I5 | 90.35 (2) |
| I2–Bi1–I5 | 174.073 (2) | I7–Bi2–I5 | 175.43 (2) |
| I3–Bi1–I4 | 90.346 (2) | I8–Bi2–I2 i | 85.10 (2) |
| I1–Bi1–I4 | 176.274 (2) | I6–Bi2–I2 i | 178.23 (2) |
| I2–Bi1–I4 | 87.516 (2) | I7–Bi2–I2 i | 88.458 (2) |
| I5–Bi1–I4 | 87.479 (2) | I5–Bi2–I2 i | 89.170 (2) |
| I3–Bi1–I4 i | 174.67 (2) | I8–Bi2–I4 | 167.43 (2) |
| I1–Bi1–I4 i | 92.564 (2) | I6–Bi2–I4 | 94.14 (2) |
| I2–Bi1–I4 i | 89.226 (2) | I7–Bi2–I4 | 90.443 (2) |
| I5–Bi1–I4 i | 87.124 (16) | I5–Bi2–I4 | 85.427 (2) |
| I4–Bi1–I4 i | 84.369 (15) | I2i–Bi2–I4 | 84.122 (2) |
| H-Bonds | D-H | H∙A (Å) | D∙∙∙A (Å) | <(DH∙∙∙A) (°) |
|---|---|---|---|---|
| N4–H4∙∙∙O1W | 0.86 | 1.96 | 2.79 (2) | 162 |
| N2–H2N∙∙∙I1 | 0.86 | 3.42 (6) | 3.89 (1) | 126 |
| N2–H2N∙∙∙I3 | 0.86 | 3.48 (8) | 3.97 (1) | 125 |
| Atoms | H | C | N | O | I | Bi |
|---|---|---|---|---|---|---|
| Surface % | 45.6 | 4 | 1.75 | 6.2 | 39.7 | 2.75 |
| H | 0.56 | - | - | - | - | - |
| C | 0.74 | 1.87 | - | - | - | - |
| N | 0.24 | - | 0.00 | - | - | - |
| O | 1.39 | - | - | 0.00 | - | - |
| I | 1.55 | 0.69 | - | 0.64 | 0.37 | - |
| Bi | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.00 |
| BFDH | ||||
| hkl | Multiplicity | Dhkl (Å) | % of TFA | |
| (0 2 0) | 2 | 12.99 | 28.84 | |
| (0 1 1) | 4 | 10.25 | 28.64 | |
| (1 1 0) | 4 | 9.84 | 25.60 | |
| (1 0 −1) | 2 | 9.49 | 9.73 | |
| (1 1 −1) | 4 | 8.92 | 7.19 | |
| Growth Morphology | ||||
| hkl | Multiplicity | dhkl (Å) | Eatt (Total) (kcal∙mol−1) | % of TFA |
| (0 2 0) | 2 | 12.99 | −32.0392 | 30.77 |
| (1 0 −1) | 2 | 9.49 | −33.1538 | 27.26 |
| (1 1 0) | 4 | 9.84 | −39.0501 | 20.81 |
| (0 1 1) | 4 | 10.25 | −51.9067 | 13.96 |
| (1 1 −1) | 4 | 8.91 | −58,9835 | 7.10 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferjani, H. Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2) 2(H2O). Crystals 2020, 10, 397. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10050397
Ferjani H. Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2) 2(H2O). Crystals. 2020; 10(5):397. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10050397
Chicago/Turabian StyleFerjani, Hela. 2020. "Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2) 2(H2O)" Crystals 10, no. 5: 397. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10050397