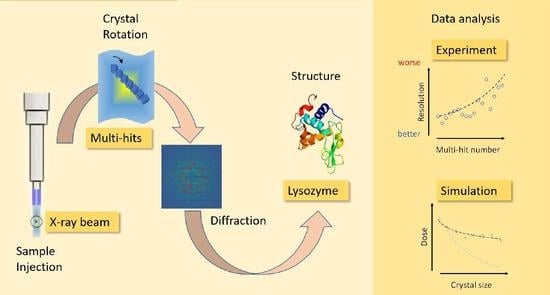
Analysis of Multi-Hit Crystals in Serial Synchrotron Crystallography Experiments Using High-Viscosity Injectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Data Collection
2.2. Identification of Single Crystals Hit Multiple Times
2.3. Radiation Dose Calculation
3. Results
3.1. Crystal Size Distribution
3.2. Radiation Dose
3.3. Model of Crystal Tumbling in the Sample Stream
3.4. Generation of Multiple Hits on a Single Crystals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SSX | Serial synchrotron Crystallography |
| WC | Whole Crystal |
| TR-SSX | Time resolved-serial synchrotron crystallography |
| SFX | Serial Femtosecond Crystallography |
| XFEL | X-ray Free Electron Laser |
References
- Mueller, C.; Marx, A.; Epp, S.; Zhong, Y.; Kuo, A.; Balo, A.; Soman, J.; Schotte, F.; Lemke, H.; Owen, R.; et al. Fixed target matrix for femtosecond time-resolved and in situ serial micro-crystallography. Struct. Dyn. 2015, 2, 054302. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xu, W.; Shi, W.; Soares, A.; Jakoncic, J.; Myers, S.; Martins, B.; Skinner, J.; Liu, Q.; Bernstein, H.; et al. High-speed raster-scanning synchrotron serial microcrystallography with a high-precision piezo-scanner. J. Synchrotron Radiat. 2018, 25, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.M.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 angstrom resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–265. [Google Scholar] [CrossRef] [PubMed]
- Wiedorn, M.O.; Oberthur, D.; Bean, R.; Schubert, R.; Werner, N.; Abbey, B.; Aepfelbacher, M.; Adriano, L.; Allahgholi, A.; Al-Qudami, N.; et al. Megahertz serial crystallography. Nat. Commun. 2018, 9, 4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yefanov, O.; Oberthur, D.; Bean, R.; Wiedorn, M.O.; Knoska, J.; Pena, G.; Awel, S.; Gumprecht, L.; Domaracky, M.; Sarrou, I.; et al. Evaluation of serial crystallographic structure determination within megahertz pulse trains. Struct. Dyn. 2019, 6. [Google Scholar] [CrossRef]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–81. [Google Scholar] [CrossRef]
- Tenboer, J.; Basu, S.; Zatsepin, N.; Pande, K.; Milathianaki, D.; Frank, M.; Hunter, M.; Boutet, S.; Williams, G.J.; Koglin, J.E.; et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 2014, 346, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Aquila, A.; Hunter, M.S.; Doak, R.B.; Kirian, R.A.; Fromme, P.; White, T.A.; Andreasson, J.; Arnlund, D.; Bajt, S.; Barends, T.R.M.; et al. Time-resolved protein nanocrystallography using an X-ray free-electron laser. Opt. Express 2012, 20, 2706–2716. [Google Scholar] [CrossRef]
- Berntsen, P.; Hadian-Jazi, M.; Kusel, M.; Martin, A.V.; Ericsson, T.; Call, M.J.; Trenker, R.; Roque, F.G.; Darmanin, C.; Abbey, B. The serial millisecond crystallography instrument at the Australian Synchrotron incorporating the “Lipidico” injector. Rev. Sci. Instruments 2019, 90, 085110. [Google Scholar] [CrossRef]
- Beyerlein, K.R.; Dierksmeyer, D.; Mariani, V.; Kuhn, M.; Sarrou, I.; Ottaviano, A.; Awel, S.; Knoska, J.; Fuglerud, S.; Jonsson, O.; et al. Mix-and-diffuse serial synchrotron crystallography. IUCrJ 2017, 4, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Gati, C.; Bourenkov, G.; Klinge, M.; Rehders, D.; Stellato, F.; Oberthuer, D.; Yefanov, O.; Sommer, B.P.; Mogk, S.; Duszenko, M.; et al. Serial crystallography on in vivo grown microcrystals using synchrotron radiation. IUCrJ 2014, 1, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, J.M.; Conrad, C.E.; Nelson, G.; Stander, N.; Zatsepin, N.A.; Zook, J.; Zhu, L.; Geiger, J.; Chun, E.; Kissick, D.; et al. Serial millisecond crystallography of membrane and soluble protein microcrystals using synchrotron radiation. IUCrJ 2017, 4, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Nogly, P.; James, D.; Wang, D.J.; White, T.A.; Zatsepin, N.; Shilova, A.; Nelson, G.; Liu, H.G.; Johansson, L.; Heymann, M.; et al. Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ 2015, 2, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Bourenkov, G.; Gati, C.; Chapman, H.N.; Redecke, L.; Zander, U.; Marquez, J.A.; Cipriani, F.; Schneider, T.R. Serial synchrotron crystallography experiments at EMBL beamline P14 at PETRA III. Acta Crystallogr. Found. Adv. 2015, 71, S12. [Google Scholar] [CrossRef] [Green Version]
- Stellato, F.; Oberthuer, D.; Liang, M.; Bean, R.; Gati, C.; Yefanov, O.; Barty, A.; Burkhardt, A.; Fischer, P.; Galli, L.; et al. Room temperature macromolecular serial crystallography using synchrotron radiation. IUCrJ 2014, 1, 204–212. [Google Scholar] [CrossRef]
- Meents, A.; Wiedorn, M.; Srajer, V.; Henning, R.; Sarrou, I.; Bergtholdt, J.; Barthelmess, M.; Reinke, P.; Dierksmeyer, D.; Tolstikova, A.; et al. Pink-beam serial crystallography. Nat. Commun. 2017, 8, 1281. [Google Scholar] [CrossRef] [Green Version]
- Botha, S.; Nass, K.; Barends, T.R.M.; Kabsch, W.; Latz, B.; Dworkowski, F.; Foucar, L.; Panepucci, E.; Wang, M.; Shoeman, R.L.; et al. Room-temperature serial crystallography at synchrotron X-ray sources using slowly flowing free-standing high-viscosity microstreams. Acta Crystallogr. Sect. D 2015, 71, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Aumonier, S.; Santoni, G.; Gotthard, G.; von Stetten, D.; Leonard, G.A.; Royant, A. Millisecond time-resolved serial oscillation crystallography of a blue-light photoreceptor at a synchrotron. IUCrJ 2020, 7, 728–736. [Google Scholar] [CrossRef]
- Weinert, T.; Skopintsev, P.; James, D.; Dworkowski, F.; Panepucci, E.; Kekilli, D.; Furrer, A.; Brunle, S.; Mous, S.; Ozerov, D.; et al. Proton uptake mechanism in bacteriorhodopsin captured by serial synchrotron crystallography. Science 2019, 365, 61. [Google Scholar] [CrossRef]
- Schulz, E.; Mehrabi, P.; Mueller-Werkmeister, H.M.; Tellkamp, F.; Jha, A.; Stuart, W.; Persch, E.; Gasparo, R.; Diederich, F.; Pai, E.; et al. The hit-and-return system enables efficient time-resolved serial synchrotron crystallography. Nat. Methods 2018, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrabi, P.; Schulz, E.; Agthe, M.; Horrell, S.; Bourenkov, G.; von Stetten, D.; Leimkohl, J.P.; Schikora, H.; Schneider, T.; Pearson, A.; et al. Liquid application method for time-resolved analyses by serial synchrotron crystallography. Nat. Methods 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, P.; Schulz, E.; Dsouza, R.; Mueller-Werkmeister, H.M.; Tellkamp, F.; Miller, R.J.D.; Pai, E.F. Time-resolved crystallography reveals allosteric communication aligned with molecular breathing. Science 2019, 365, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Baek, S.; Park, J.; Lee, K.; Kim, J.; Lee, S.J.; Chung, W.K.; Lee, J.L.; Cho, Y.; Nam, K.H. Nylon mesh-based sample holder for fixed-target serial femtosecond crystallography. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.V.; Kozlov, A.; Berntsen, P.; Roque, F.G.; Flueckiger, L.; Saha, S.; Greaves, T.L.; Conn, C.E.; Hawley, A.M.; Ryan, T.M. Fluctuation X-ray diffraction reveals three-dimensional nanostructure and disorder in self-assembled lipid phases. Commun. Mater. 2020, 1, 1–8. [Google Scholar]
- Roedig, P.; Vartiainen, I.; Duman, R.; Panneerselvam, S.; Stube, N.; Lorbeer, O.; Warmer, M.; Sutton, G.; Stuart, D.I.; Weckert, E.; et al. A micro-patterned silicon chip as sample holder for macromolecular crystallography experiments with minimal background scattering. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Shelby, M.; Gilbile, D.; Grant, T.; Seuring, C.; Segelke, B.; He, W.; Evans, A.; Pakendorf, T.; Fischer, P.; Hunter, M.; et al. Fixed Target Delivery for Serial Femtosecond Crystallography of Weakly-Diffracting Objects. Protein Sci. 2019, 28, 109–110. [Google Scholar]
- Abbey, B.; Dilanian, R.A.; Darmanin, C.; Ryan, R.A.; Putkunz, C.T.; Martin, A.V.; Wood, D.; Streltsov, V.; Jones, M.W.M.; Gaffney, N.; et al. X-ray laser-induced electron dynamics observed by femtosecond diffraction from nanocrystals of Buckminsterfullerene. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- Martiel, I.; Müller-Werkmeister, H.M.; Cohen, A.E. Strategies for sample delivery for femtosecond crystallography. Acta Crystallogr. Sect. D 2019, 75, 160–177. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, P.; Müller-Werkmeister, H.M.; Leimkohl, J.P.; Schikora, H.; Ninkovic, J.; Krivokuca, S.; Andriček, L.; Epp, S.W.; Sherrell, D.; Owen, R.L.; et al. The HARE chip for efficient time-resolved serial synchrotron crystallography. J. Synchrotron Radiat. 2020, 27, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Conrad, C.E.; Basu, S.; James, D.; Wang, D.J.; Schaffer, A.; Roy-Chowdhury, S.; Zatsepin, N.A.; Aquila, A.; Coe, J.; Gati, C.; et al. A novel inert crystal delivery medium for serial femtosecond crystallography. IUCrJ 2015, 2, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Kim, J.; Park, G.; Cho, Y.; Nam, K.H. Polyacrylamide injection matrix for serial femtosecond crystallography. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, M.; Song, C.Y.; Suzuki, M.; Masuda, T.; Inoue, S.; Nakane, T.; Yumoto, F.; Nango, E.; Tanaka, R.; Tono, K.; et al. Oil-free hyaluronic acid matrix for serial femtosecond crystallography. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, M.; Mizohata, E.; Nango, E.; Suzuki, M.; Tanaka, T.; Masudala, T.; Tanaka, R.; Shimamura, T.; Tanaka, Y.; Suno, C.; et al. Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 2015, 12, 61–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovácsová, G.; Grünbein, M.L.; Kloos, M.; Barends, T.R.M.; Schlesinger, R.; Heberle, J.; Kabsch, W.; Shoeman, R.L.; Doak, R.B.; Schlichting, I. Viscous hydrophilic injection matrices for serial crystallography. IUCrJ 2017, 4, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, M.; Nakane, T.; Masuda, T.; Suzuki, M.; Inoue, S.; Song, C.; Tanaka, R.; Nakatsu, T.; Mizohata, E.; Yumoto, F.; et al. Hydroxyethyl cellulose matrix applied to serial crystallography. Sci. Rep. 2017, 7, 703. [Google Scholar] [CrossRef] [PubMed]
- Standfuss, J. Membrane protein dynamics studied by X-ray lasers—Or why only time will tell. Curr. Opin. Struct. Biol. 2019, 57, 63–71. [Google Scholar] [CrossRef]
- DePonte, D.P.; Weierstall, U.; Schmidt, K.; Warner, J.; Starodub, D.; Spence, J.C.H.; Doak, R.B. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D-Appl. Phys. 2008, 41. [Google Scholar] [CrossRef] [Green Version]
- Oberthuer, D.; Knoska, J.; Wiedorn, M.O.; Beyerlein, K.R.; Bushnell, D.A.; Kovaleva, E.G.; Heymann, M.; Gumprecht, L.; Kirian, R.A.; Barty, A.; et al. Double-flow focused liquid injector for efficient serial femtosecond crystallography. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Ganan-Calvo, A.M. Generation of steady liquid microthreads and micron-sized monodisperse sprays in gas streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Zhu, L.; Weierstall, U.; Cherezov, V.; Liu, W. Serial Femtosecond Crystallography of Membrane Proteins. Next Gener. Membr. Protein Struct. Determ. 2016, 922, 151–160. [Google Scholar]
- Berntsen, P.; Sharma, R.; Kusel, M.; Abbey, B.; Darmanin, C. Lipidico Injection Protocol for Serial Crystallography Measurements at the Australian Synchrotron. J. Vis. Exp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hadian-Jazi, M.; Messerschmidt, M.; Darmanin, C.; Giewekemeyer, K.; Mancuso, A.P.; Abbey, B. A peak-finding algorithm based on robust statistical analysis in serial crystallography. J. Appl. Crystallogr. 2017, 50, 1705–1715. [Google Scholar] [CrossRef]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, J.L.; McCubbin, P.T.N.; Garman, E.F. RADDOSE-XFEL: Femtosecond time-resolved dose estimates for macromolecular X-ray free-electron laser experiments. J. Appl. Crystallogr. 2020, 53, 549–560. [Google Scholar] [CrossRef]
- Marman, H.; Darmanin, C.; Abbey, B. The Influence of Photoelectron Escape in Radiation Damage Simulations of Protein Micro-Crystallography. Crystals 2018, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- de la Mora, E.; Coquelle, N.; Bury, C.S.; Rosenthal, M.; Holton, J.M.; Carmichael, I.; Garman, E.F.; Burghammer, M.; Colletier, J.P.; Weik, M. Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures. Proc. Natl. Acad. Sci. USA 2020, 117, 4142–4151. [Google Scholar] [CrossRef] [Green Version]
- Nogly, P.; Panneels, V.; Nelson, G.; Gati, C.; Kimura, T.; Milne, C.; Milathianaki, D.; Kubo, M.; Wu, W.; Conrad, C.; et al. Lipidic cubic phase injector is a viable crystal delivery system for time-resolved serial crystallography. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Cohen, A.E.; Soltis, S.M.; González, A.; Aguila, L.; Alonso-Mori, R.; Barnes, C.O.; Baxter, E.L.; Brehmer, W.; Brewster, A.S.; Brunger, A.T.; et al. Goniometer-based femtosecond crystallography with X-ray free electron lasers. Proc. Natl. Acad. Sci. USA 2014, 111, 17122–17127. [Google Scholar] [CrossRef] [Green Version]
- Roessler, C.; Agarwal, R.; Allaire, M.; Alonso-Mori, R.; Andi, B.; Bachega, J.; Bommer, M.; Brewster, A.; Browne, M.; Chatterjee, R.; et al. Acoustic Injectors for Drop-On-Demand Serial Femtosecond Crystallography. Structure 2016, 24, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.; Owen, R. Combining X-ray crystallography and single-crystal spectroscopy to probe enzyme mechanisms. Biochem. Soc. Trans. 2009, 37, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Khakurel, K.P.; Espinoza, S.; Savko, M.; Polovinkin, V.; Dohnalek, J.; Shepard, W.; Angelova, A.; Hajdu, J.; Andreasson, J.; Angelov, B. Kilohertz Macromolecular Crystallography Using an EIGER Detector at Low X-ray Fluxes. Crystals 2020, 12, 1146. [Google Scholar] [CrossRef]
- Southworth-Davies, R.J.; Medina, M.A.; Carmichael, I.; Garman, E.F. Observation of decreased radiation damage at higher dose rates in room temperature protein crystallography. Structure 2007, 15, 1531–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nave, C.; Garman, E.F. Towards an understanding of radiation damage in cryocooled macromolecular crystals. J. Synchrotron Radiat. 2005, 12, 257–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotthard, G.; Aumonier, S.; De Sanctis, D.; Leonard, G.; von Stetten, D.; Royant, A. Specific radiation damage is a lesser concern at room temperature. IUCrJ 2019, 6, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Data Parameters | Lysozyme * | Multi-Hit Dataset |
|---|---|---|
| Diffraction source | Australian Synchrotron | Australian Synchrotron |
| Photon Energy (mean value, eV) | 13,000 | 13,000 |
| Flux (photons/s) | 2.4 × 1012 | 2.4 × 1012 |
| Wavelength (Å) | 1.05 | 1.05 |
| Temperature (°K) | 300 | 300 |
| Detector | Dectris EIGER X 16M | Dectris EIGER X 16M |
| Beam size (W,H μm) | 12 × 22 | 12 × 22 |
| Space group | P43212 | P43212 |
| a, b, c (Å) | 76.68, 76.68, 38.48 | 76.68, 76.68, 38.48 |
| , , , (°) | 90, 90, 90 | 90, 90, 90 |
| Resolution range (Å) | 78.68–1.83 (2.05–1.83) | 34–2.2 (7.96–2.2) |
| Total number frames | 224,200 | 4389 |
| No. of crystals | 4794 | 315 |
| No. total reflections | 1,394,451 | 18,191 (2267) |
| No. of unique reflections | 6614 | 1294 (159) |
| Completeness (%) | 99.44 (99.15) | 8.30 (7.9) |
| Redundancy | 73.97 (51.34) | 14.06 (11.0) |
| I/ (I) | 5.08 (1.30) | 3.30 (1.54) |
| CC1/2 | 0.96 (0.47) | 0.90 (0.68) |
| CC * | 0.99 (0.81) | 0.99 (0.8) |
| Rsplit | 14.09 (93.57) | 14.52 (63.71) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadian-Jazi, M.; Berntsen, P.; Marman, H.; Abbey, B.; Darmanin, C. Analysis of Multi-Hit Crystals in Serial Synchrotron Crystallography Experiments Using High-Viscosity Injectors. Crystals 2021, 11, 49. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010049
Hadian-Jazi M, Berntsen P, Marman H, Abbey B, Darmanin C. Analysis of Multi-Hit Crystals in Serial Synchrotron Crystallography Experiments Using High-Viscosity Injectors. Crystals. 2021; 11(1):49. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010049
Chicago/Turabian StyleHadian-Jazi, Marjan, Peter Berntsen, Hugh Marman, Brian Abbey, and Connie Darmanin. 2021. "Analysis of Multi-Hit Crystals in Serial Synchrotron Crystallography Experiments Using High-Viscosity Injectors" Crystals 11, no. 1: 49. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010049