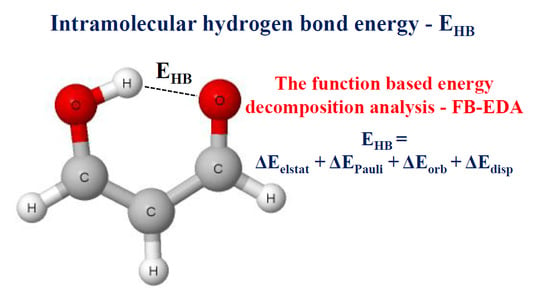
Intramolecular Hydrogen Bond Energy and Its Decomposition—O–H∙∙∙O Interactions
Abstract
:1. Introduction
- -
- The analysis of intraHBs in simple species; malonaldehyde and its fluoro-derivatives.
- -
- The comparison of the above species with similar types in crystal structures.
- -
- The critical analysis of the estimation of intraHB energy that is based on comparison of the conformation containing this interaction with conformations that do not possess it.
- -
- The proposal of the new method to calculate intraHB energy. Following steps may be indicated here: (1) the choice of structures with intraHBs for which such energies are to be calculated; (2) finding of relationships between HB energy and other parameters for related complexes linked by intermolecular hydrogen bonds; (3) the application of the latter relationships for intraHBs being the subject of analysis.
- -
- The comparison of the above new method with other approaches.
- -
- Additionally, the method of the decomposition of energy of interaction for intraHB systems is proposed in this study, apparently for the first time.
2. Models to Calculate Energies of Intramolecular Hydrogen Bonds
3. Computational Methods
4. Results and Discussion
4.1. Characteristics of Intramolecular Hydrogen Bonds
4.2. Quantum Theory of ‘Atoms in Molecules’ Parameters
4.3. Intramolecular Hydrogen Bonds and the Choice of Reference Systems
4.4. Related Intermolecular Hydrogen Bonds
4.5. The New Method of the Evaluation of the Intramolecular Hydrogen Bond Energy—Estimations Based on Relationships for Intermolecular Interactions
4.6. The Function-Based Decomposition of the Intramolecular Hydrogen Bond Energy
ΔEelstat = −527.76 ρBCP − 7.3363 (R2 = 0.9801)
ΔEorb = −377.7 ρBCP − 6.2543 (R2 = 0.9896)
ΔEdisp = −34.027 ρBCP − 0.2344 (R2 = 0.2695)
5. Conclusions
- -
- the choice of the sample of systems with intramolecular hydrogen bonds that are to be analyzed, and the performing calculations for the species of this sample;
- -
- the choice of the sample of complexes linked by intermolecular hydrogen bonds and the performing calculations for them, it is important that these complexes are characterized by the same arrangements as those occurring for intramolecular hydrogen bonds;
- -
- the application of the relationships between the hydrogen bond energy and other parameters found for the sample of complexes to evaluate the energies of intramolecular hydrogen bonds.
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Sidgwick, N.V.; Callow, R.K. Abnormal Benzene Derivatives. J. Chem. Soc. 1924, 125, 527–538. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Buemi, G. Ab initio study of 2,4-dihalosubstituted malonaldehyde and 2-halo-phenols in gas phase and solution. Chem. Phys. 2002, 277, 241–256. [Google Scholar] [CrossRef]
- Buemi, G. Basis set effects on the energy of intramolecular O-H∙∙∙halogen hydrogen bridges in ortho-halophenols and 2,4-dihalo-malonaldehyde. Chem. Phys. 2004, 300, 107–117. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Cryst. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Cheng, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Gilli, G.; Bellucci, F.; Ferretti, V.; Bertolasi, V. Evidence for Resonance-Assisted Hydrogen Bonding from Crystal-Structure Correlations on the Enol Form of the β-Diketone Fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Evidence for Resonance-Assisted Hydrogen Bonding. 2. Intercorrelation between Crystal Structure and Spectroscopic Parameters in Eight Intramolecularly Hydrogen Bonded 1,3-Diaryl-1,3-propanedione Enols. J. Am. Chem. Soc. 1991, 113, 4917–4925. [Google Scholar] [CrossRef]
- Cuma, M.; Scheiner, S.; Kar, T. Effect of adjoining aromatic ring upon excited state proton transfer, o-hydroxybenzaldehyde. J. Mol. Struct. (Theochem) 1999, 467, 37–49. [Google Scholar] [CrossRef]
- Korth, H.G.; de Heer, M.I.; Mulder, P. A DFT Study on Intramolecular Hydrogen Bonding in 2-Substituted Phenols: Conformations, Enthalpies, and Correlation with Solute Parameters. J. Phys. Chem. A 2002, 106, 8779–8789. [Google Scholar] [CrossRef]
- Woodford, J.N. Density Functional Theory and Atoms-in-Molecules Investigation of Intramolecular Hydrogen Bonding in Derivatives of Malonaldehyde and Implications for Resonance-Assisted Hydrogen Bonding. J. Phys. Chem. A 2007, 111, 8519–8530. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Mó, O.; Yañez, M.; Del Bene, J.E. Do coupling constants and chemical shifts provide evidence for the existence of resonance-assisted hydrogen bonds? Mol. Phys. 2004, 102, 2563–2574. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Mó, O.; Yañez, M.; Del Bene, J.E. Are resonance-assisted hydrogen bonds ´resonance assisted´? A theoretical NMR study. Chem. Phys. Lett. 2005, 411, 411–415. [Google Scholar] [CrossRef]
- Góra, R.W.; Maj, M.; Grabowski, S.J. Resonance-assisted hydrogen bonds revisited. Resonance stabilization vs. charge delocalization. Phys. Chem. Chem. Phys. 2013, 15, 2514–2522. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Romero-Montalvo, E.; Costales, A.; Pendás, A.M.; Rocha-Rinza, T. The nature of resonance-assisted hydrogen bonds: A quantum chemical topology perspective. Phys. Chem. Chem. Phys. 2016, 18, 26383–26390. [Google Scholar] [CrossRef] [Green Version]
- Kopylovich, M.N.; Mahmudov, K.T.; Mizar, A.; Pombeiro, A.J.L. Hydrogen bond assisted activation of a dinitrile towards nucleophilic attack. Chem. Commun. 2011, 47, 7248–7250. [Google Scholar] [CrossRef]
- Su, X.; Lõkov, M.; Kütt, A.; Leito, I.; Aprahamian, I. Unusual para-substituent effects on the intramolecular hydrogen-bond in hydrazone-based switches. Chem. Commun. 2012, 48, 10490–10492. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, A.; Paul, S.; Das, A.K.; Aich, K.; Nandi, P.K. Resonance-assisted hydrogen bonding induced nucleophilic addition to hamper ESIPT: Ratiometric detection of cyanide in aqueous media. Chem. Commun. 2013, 49, 2912–2914. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W. A new fluorescent probe for gasotransmitter H2S: High sensitivity, excellent selectivity, and a significant luorescence off–on response. Chem. Commun. 2014, 50, 4214–4217. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Pombeiro, A.J.L. Resonance-Assisted Hydrogen Bonding as Driving Force in Synthesis and a Synthon in the Design of Materials. Chem. Eur. J. 2016, 22, 16356–16398. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Del Bene, J.E. What factors determine whether a proton-bound homodimer has a symmetric or an asymmetric hydrogen bond? Mol. Phys. 2009, 107, 1095–1105. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Iversen, B.B.; Larsen, F.K.; Kapon, M.; Reisner, G.M.; Herbstein, F.-H. Topological Analysis of the Charge Density in Short Intramolecular O-H∙∙∙O Hydrogen Bonds. Very Low Temperature X-ray and Neutron Diffraction Study of Benzoylacetone. J. Am. Chem. Soc. 1998, 120, 10040–10045. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; McIntyre, G.J.; Schiøtt, B.; Larsen, F.K. The Low-Barrier Hydrogen Bond of Deuterated Benzoylacetone Probed by Very Low Temperature Neutron and X-ray Diffraction Studies and Theoretical Calculations. Chem. Eur. J. 2007, 13, 5539–5547. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W. Low-Barrier Hydrogen Bonds and Low Fractionation Factor Bases in Enzymatic Reactions. Biochemistry 1992, 31, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Nielson, J.B. “Strong” Hydrogen Bonds in Chemistry and Biology. Annu. Rev. Phys. Chem. 1997, 48, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Hosur, M.V.; Chitra, R.; Hegde, S.; Choudhury, R.R.; Das, A.; Hosur, R.V. Low-barrier hydrogen bonds in proteins. Crystallogr. Rev. 2013, 19, 3–50. [Google Scholar] [CrossRef]
- Alder, R.W. Strain effects on amine basicities. Chem. Rev. 1989, 89, 1215–1223. [Google Scholar] [CrossRef]
- Staab, H.A.; Saupe, T. “Proton sponges” and the geometry of hydrogen bonds: Aromatic nitrogen bases with exceptional basicities. Angew. Chem. Int. Ed. Engl. 1988, 27, 865–879. [Google Scholar] [CrossRef]
- López, C.; Lorente, P.; Claramunt, R.M.; Marín, J.; Foces-Foces, C.; Llamas-Saiz, A.L.; Elguero, J.; Limbach, H.-H. Localization of hydrogen bond deuterons in proton sponges by dipolar solid state 15N NMR spectroscopy. Ber. Bunsenges. Phys. Chem. 1998, 102, 414–418. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Sigalov, M.; Shenderovich, I.G.; Mulloyarova, V.V.; Denisov, G.S.; Tolstoy, P.M. Correlations of NHN hydrogen bond energy with geometry and 1H NMR chemical shift difference of NH protons for aniline complexes. J. Chem. Phys. 2019, 150, 114305. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. [Google Scholar] [CrossRef] [PubMed]
- Luth, K.; Scheiner, S. Excited-state energetics and proton-transfer barriers in malonaldehyde. J. Phys. Chem. 1994, 98, 3582–3587. [Google Scholar] [CrossRef]
- Latajka, Z.; Scheiner, S. Proton transfer in the ground and first excited triplet states of malonaldehyde. J. Phys. Chem. 1992, 96, 9764–9767. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E. Retrieving interaction potentials from the topology of the electron density distribution: The case of hydrogen bonds. J. Chem. Phys. 2000, 113, 5686–5694. [Google Scholar] [CrossRef]
- Jabłoński, M.; Kaczmarek, A.; Sadlej, A. Estimates of the energy of intramolecular hydrogen bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef]
- Musin, R.N.; Mariam, Y.H. An integrated approach to the study of intramolecular hydrogen bonds in malonaldehyde enol derivatives and naphthazarin: Trend in energetic versus geometrical consequences. J. Phys. Org. Chem. 2006, 19, 425–444. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: Oxford, UK, 2009; pp. 75–81. [Google Scholar]
- Babu, K.; Gadre, S.R.; Ghermani, N.E. Tailoring approach for exploring electron densities and electrostatic potentials of molecular crystals. Theor. Chem. Acc. 2004, 111, 255–263. [Google Scholar] [CrossRef]
- Ganesh, V.; Dongare, R.K.; Balanarayan, P.; Gadre, S.R. Molecular tailoring approach for geometry optimization of large molecules: Energy evaluation and parallelization strategies. J. Chem. Phys. 2006, 125, 104109. [Google Scholar] [CrossRef]
- Rusinska-Roszak, D.; Sowinski, G. Estimation of the Intramolecular O–H···O=C Hydrogen Bond Energy via the Molecular Tailoring Approach. Part I: Aliphatic Structures. J. Chem. Inf. Model. 2014, 54, 1963–1977. [Google Scholar] [CrossRef]
- Rusinska-Roszak, D. Intramolecular O–H···O=C Hydrogen Bond Energy via the Molecular Tailoring Approach to RAHB Structures. J. Phys. Chem. A 2015, 119, 3674–3687. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V. Benchmark calculations of intramolecular hydrogen bond energy based on molecular tailoring and function-based approaches: Developing hybrid approach. Int. J. Quantum Chem. 2019, 119, e26001. [Google Scholar] [CrossRef]
- Afonin, A.V.; Pavlov, D.V.; Vashchenko, A.V. Case study of 2-vinyloxypyridine: Quantitative assessment of the intramolecular C-H⋯N hydrogen bond energy and its contribution to the one-bond 13C-1H coupling constant. J. Mol. Struct. 2019, 1176, 73–85. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V. Quantitative decomposition of resonance-assisted hydrogen bond energy in β-diketones into resonance and hydrogen bonding (π- and σ-) components using molecular tailoring and function-based approaches. J. Comput. Chem. 2020, 41, 1285–1298. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. Bridging QTAIM with vibrational spectroscopy: The energy of intramolecular hydrogen bonds in DNA-related biomolecules. Phys. Chem. Chem. Phys. 2012, 14, 7441–7447. [Google Scholar] [CrossRef]
- Brovarets, O.O.; Hovorun, D.M. Intramolecular tautomerization of the quercetin molecule due to the proton transfer: QM computational study. PLoS ONE 2019, 14, e0224762. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Minenkov, Y.; Singstad, A.; Occhipinti, G.; Jensen, V.R. The accuracy of DFT-optimized geometries of functional transition metal compounds: A validation study of catalysts for olefin metathesis and other reactions in the homogeneous phase. Dalton Trans. 2012, 41, 5526–5541. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Burns, L.A.; Vázquez-Mayagoitia, A.; Sumpter, B.G.; Sherrill, C.D. Density-functional approaches to noncovalent interactions: A comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J. Chem. Phys. 2011, 134, 084107. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–561. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, a Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Matta, C.; Boyd, R.J. (Eds.) Quantum Theory of Atoms in Molecules: Recent Progress in Theory and Application; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Keith, T.A. AIMAll, version 11.08.23; TK Gristmill Software: Overland Park, KS, USA, 2011. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1-118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. CO, CS, N2, PF3, and CNCH3 as σ Donors and π Acceptors. A Theoretical Study by the Hartree-Fock-Slater Transition-State Method. Inorg. Chem. 1979, 18, 1755–1759. [Google Scholar] [CrossRef]
- Velde, G.T.E.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF2013, SCM; Theoretical Chemistry, Vrije Universiteit: Amsterdam, The Netherlands, 2013; Available online: http://www.scm.com/news/adf2013-modeling-suite-released/ (accessed on 16 September 2014).
- Grabowski, S.J. An estimation of strength of intramolecular hydrogen bonds—Ab initio and AIM studies. J. Mol. Struct. 2001, 562, 137–143. [Google Scholar] [CrossRef]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-Bond and Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Allen, F.H.; Willett, P. The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Cryst. 2010, 43, 811–824. [Google Scholar] [CrossRef] [Green Version]
- Luger, P. Modern X-ray Analysis on Single Crystals, 2nd fully revised and extended edition; Walter de Gruyter: Berlin, Germany, 2014. [Google Scholar]
- Grabowski, S.J. Understanding Hydrogen Bonds, Theoretical and Experimental Views; The Royal Society of Chemistry: Cambridge, UK, 2021. [Google Scholar]
- Kawaguchi, K.; Hirota, E. Infrared diode laser study of the hydrogen bifluoride anion: FHF- and FDF-. J. Chem. Phys. 1986, 84, 2953–2960. [Google Scholar] [CrossRef]
- Pylaeva, S.A.; Elgabarty, H.; Sebastiani, D.; Tolstoy, P.M. Symmetry and dynamics of FHF- anion in vacuum, in CD2Cl2 and in CCl4. Ab initio MD study of fluctuating solvent–solute hydrogen and halogen bonds. Phys. Chem. Chem. Phys. 2017, 19, 26107–26120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, S.J. [FHF]-—The Strongest Hydrogen Bond under the Influence of External Interactions. Crystals 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Katagiri, K.; Azumaya, I.; Harayama, T. Trifluoroacetylation-Induced Houben-Hoesch-Type Cyclization of Cyanoacetanilides: Increased Nucleophilicity of CN Groups. J. Org. Chem. 2010, 75, 2741–2744. [Google Scholar] [CrossRef]
- Basheer, A.; Mishima, M.; Rappoport, Z. Enols of 2-nitro- and related 2-substituted malonamides. J. Phys. Org. Chem. 2010, 23, 255–265. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Jenkins, S.; Morrison, I. The chemical character of the intermolecular bonds of seven phases of ice as revealed by ab initio calculation of electron densities. Chem. Phys. Lett. 2000, 317, 97–102. [Google Scholar] [CrossRef]
- Grabowski, S.J. Properties of a ring critical point as measures of intramolecular H-bond strength. Monatsh. Chem. 2002, 133, 1373–1380. [Google Scholar] [CrossRef]
- Rybarczyk-Pirek, A.J.; Grabowski, S.J.; Małecka, M.; Nawrot-Modranka, J. Crystal and Molecular Structures of New Chromone Derivatives as Empirical Evidence of Intramolecular Proton Transfer Reaction; Ab Initio Studies on Intramolecular H-Bonds in Enaminones. J. Phys. Chem. A 2002, 106, 11956–11962. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Małecka, M. Intramolecular H-bonds: DFT and QTAIM studies on 3-(aminomethylene) pyran-2, 4-dione and its derivatives. J. Phys. Chem. A 2006, 110, 11847–11854. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Hydrogen Bonding; A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Grabowski, S.J.; Sokalski, W.A.; Dyguda, E.; Leszczynski, J. Quantitative classification of covalent and noncovalent H-bonds. J. Phys. Chem. B 2006, 110, 6444–6446. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C. Valency and Bonding, a Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds—Mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef]








| Species | O–H | H…O | O…O | OHO |
|---|---|---|---|---|
| Malonaldehyde | 0.991 | 1.690 | 2.575 | 146.3 |
| M-1 | 1.026 | 1.533 | 2.478 | 150.5 |
| M-2 | 0.984 | 1.754 | 2.619 | 144.5 |
| M-3 | 0.980 | 1.807 | 2.651 | 142.5 |
| M-12 | 1.012 | 1.598 | 2.519 | 148.8 |
| M-13 | 0.991 | 1.724 | 2.596 | 144.6 |
| M-23 | 0.976 | 1.848 | 2.678 | 141.0 |
| M-123 | 0.986 | 1.762 | 2.620 | 143.4 |
| Species | ρBCP | ∇2ρBCP | VBCP | GBCP | HBCP |
|---|---|---|---|---|---|
| Malonaldehyde | 0.051 | 0.116 | −0.052 | 0.040 | −0.011 |
| M-1 | 0.076 | 0.103 | −0.085 | 0.055 | −0.029 |
| M-2 | 0.044 | 0.113 | −0.042 | 0.035 | −0.007 |
| M-3 | 0.038 | 0.111 | −0.035 | 0.031 | −0.004 |
| M-12 | 0.064 | 0.110 | −0.069 | 0.048 | −0.021 |
| M-13 | 0.047 | 0.114 | −0.045 | 0.037 | −0.008 |
| M-23 | 0.034 | 0.107 | −0.030 | 0.029 | −0.002 |
| M-123 | 0.042 | 0.113 | −0.040 | 0.034 | −0.006 |
| Species | 1 | 2 | 3 | HH | 4 |
|---|---|---|---|---|---|
| Malonaldehyde | −11.8 | −10.9 | −16.7 | −13.6 | −13.2 |
| M-1 | −22.6 | −18.5 | −21.5 | −20.6 | −18.3 |
| M-2 | −8.7 | −8.9 | −14.0 | −13.3 | −11.9 |
| M-3 | −9.0 | −9.7 | −12.9 | −11.6 | −15.2 |
| M-12 | −18.1 | −15.7 | −18.1 | −19.2 | −15.9 |
| M-13 | −15.0 | −19.9 | −14.0 | −15.1 | −17.3 |
| M-23 | −6.7 | −7.4 | −11.1 | −9.7 | −12.8 |
| M-123 | −12.5 | −15.6 | −12.2 | −12.8 | −14.6 |
| Complex | Eint | EintBSSE | Eint (Equation (1)) |
|---|---|---|---|
| 1 | −8.4 | −8.2 | −10.2 |
| 2 | −12.6 | −12.3 | −14.0 |
| 3 | −9.4 | −9.2 | −11.0 |
| 4 | −7.9 | −7.7 | −10.1 |
| 5 | −6.5 | −6.3 | −8.6 |
| 6 | −12.2 | −12.0 | −14.0 |
| 7 | −11.8 | −11.5 | −13.8 |
| 8 | −8.8 | −8.7 | −11.2 |
| 9 | −11.8 | −11.6 | −13.5 |
| Complex | ΔEPauli | ΔEelstat | ΔEorb | ΔEdisp | ΔEint |
|---|---|---|---|---|---|
| 1 | 10.9 | −11.1 | −7.1 | −1.2 | −8.5 |
| 2 | 16.6 | −16.8 | −11.1 | −2.0 | −13.3 |
| 3 | 13.7 | −13.5 | −8.2 | −2.1 | −10.1 |
| 4 | 10.8 | −10.7 | −7.0 | −1.2 | −8.1 |
| 5 | 9.5 | −9.2 | −5.6 | −1.4 | −6.6 |
| 6 | 15.7 | −16.3 | −10.6 | −1.5 | −12.8 |
| 7 | 16.4 | −16.1 | −10.8 | −2.0 | −12.5 |
| 8 | 12.5 | −12.6 | −7.7 | −1.5 | −9.3 |
| 9 | 15.1 | −15.8 | −10.2 | −1.5 | −12.4 |
| Species | EHB* | EHB (Equation (1)) | EHB** | EHB (Equation (3)) | EHB (Equation (4)) | EHB (Equation (2)) |
|---|---|---|---|---|---|---|
| Malonaldehyde | −14.4 | −16.2 | −13.9 | −13.7 | −4.8 | −11.4 |
| M-1 | −24.4 | −26.5 | −24.5 | −20.5 | −9.0 | −20.6 |
| M-2 | −11.4 | −13.1 | −11.1 | −11.4 | −3.6 | −8.8 |
| M-3 | −9.1 | −10.9 | −9.1 | −10.0 | −2.9 | −9.4 |
| M-12 | −19.7 | −21.5 | −19.4 | −17.3 | −6.9 | −16.9 |
| M-13 | −12.5 | −14.2 | −12.3 | −12.6 | −4.1 | −17.5 |
| M-23 | −7.6 | −9.4 | −7.9 | −9.0 | −2.4 | −7.0 |
| M-123 | −10.8 | −12.5 | −10.7 | −11.4 | −3.5 | −14.0 |
| Species | ΔEPauli | ΔEelstat | ΔEorb | ΔEint − ΔEdisp | ΔEint |
|---|---|---|---|---|---|
| Malonaldehyde | 19.0 | −19.6 | −13.0 | −13.6 | −15.2 |
| M-1 | 31.0 | −32.6 | −22.3 | −23.9 | −25.5 |
| M-2 | 15.5 | −15.7 | −10.3 | −10.5 | −12.1 |
| M-3 | 12.7 | −12.8 | −8.1 | −8.2 | −9.8 |
| M-12 | 25.4 | −26.5 | −18.0 | −19.1 | −20.7 |
| M-13 | 16.8 | −17.2 | −11.3 | −11.7 | −13.3 |
| M-23 | 10.9 | −10.8 | −6.7 | −6.6 | −8.2 |
| M-123 | 14.7 | −4.9 | −9.7 | −9.9 | −11.5 |
| Species | 1 | 2 | 3 | HH | 4 |
|---|---|---|---|---|---|
| Malonaldehyde | −9.1 (2.8) | −8.2 (2.8) | −12.8 (3.8) | −9.7 (4.0) | −10.1 (3.1) |
| M-1 | −14.1 (8.5) | −12.2 (6.3) | −14.1 (7.3) | −12.5 (8.1) | −12.1 (6.2) |
| M-2 | −6.4 (2.3) | −6.5 (2.3) | −10.8 (3.2) | −9.8 (3.5) | −9.4 (2.5) |
| M-3 | −6.9 (2.1) | −7.2 (2.5) | −10.2 (2.7) | −4.5 (7.0) | −10.6 (4.6) |
| M-12 | −11.9 (6.2) | −11.0 (4.7) | −12.4 (5.7) | −12.4 (6.8) | −11.3 (4.6) |
| M-13 | −10.5 (4.6) | −11.1 (8.8) | −10.5 (3.5) | −5.7 (9.4) | −11.1 (6.2) |
| M-23 | −4.9 (1.8) | −4.9 (2.5) | −9.0 (2.1) | −4.1 (5.6) | −9.0 (3.8) |
| M-123 | −9.1 (3.4) | −9.3 (6.3) | −9.5 (2.7) | −5.2 (7.6) | −9.7 (4.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, S.J. Intramolecular Hydrogen Bond Energy and Its Decomposition—O–H∙∙∙O Interactions. Crystals 2021, 11, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010005
Grabowski SJ. Intramolecular Hydrogen Bond Energy and Its Decomposition—O–H∙∙∙O Interactions. Crystals. 2021; 11(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010005
Chicago/Turabian StyleGrabowski, Sławomir J. 2021. "Intramolecular Hydrogen Bond Energy and Its Decomposition—O–H∙∙∙O Interactions" Crystals 11, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010005