New Low-Dimensional Hybrid Perovskitoids Based on Lead Bromide with Organic Cations from Charge-Transfer Complexes
Abstract
:1. Introduction
2. Materials and Methods
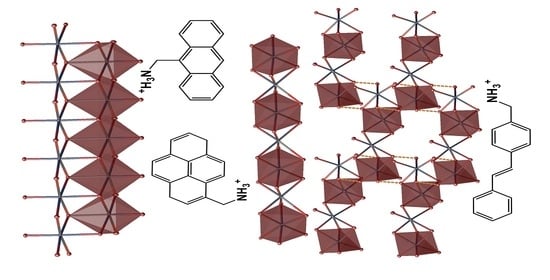
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, M.; Zhou, X.; Wu, J.; Zhang, L.; Kong, W.; Li, X.; Zhao, X.; Dai, S.; Xu, B.; et al. Efficiency and stability enhancement of perovskite solar cells by introducing CsPbI3 quantum dots as an interface engineering layer. NPG Asia Mater. 2018, 10, 552–561. [Google Scholar] [CrossRef]
- Huo, C.; Cai, B.; Yuan, Z.; Ma, B.; Zeng, H. Two-Dimensional Metal Halide Perovskites: Theory, Synthesis, and Optoelectronics. Small Methods 2017, 1, 1600018. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Worku, M.; Neu, J.; Lin, H.; Tian, Y.; Lee, S.; Zhou, Y.; Han, D.; Chen, S.; Hao, A.; et al. Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency. Chem. Mat. 2018, 30, 2374–2378. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An extended Tolerance Factor approach for organic–inorganic perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [Green Version]
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. [Google Scholar] [CrossRef]
- Mousdis, G.A.; Gionis, V.; Papavassiliou, G.C.; Raptopoulou, C.P.; Terzis, A. Preparation, structure and optical properties of [CH3SC(=NH2)NH2]3PbI5, [CH3SC(=NH2)NH2]4Pb2Br8 and [CH3SC(=NH2)NH2]3PbCl5·CH3SC(=NH2)NH2CL. J. Mat. Chem. 1998, 8, 2259–2262. [Google Scholar] [CrossRef]
- Rahaman, M.Z.; Ge, S.; Lin, C.-H.; Cui, Y.; Wu, T. One-Dimensional Molecular Metal Halide Materials: Structures, Properties, and Applications. Small Struct. 2021, 2, 2000062. [Google Scholar] [CrossRef]
- Sun, S.; Lu, M.; Gao, X.; Shi, Z.; Bai, X.; Yu, W.W.; Zhang, Y. 0D Perovskites: Unique Properties, Synthesis, and Their Applications. Adv. Sci. 2021, 2102689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; Lee, S.; Chaaban, M.; Ma, B. Organic–inorganic metal halide hybrids beyond perovskites. Mat. Res. Lett. 2018, 6, 552–569. [Google Scholar] [CrossRef] [Green Version]
- Passarelli, J.V.; Fairfield, D.J.; Sather, N.A.; Hendricks, M.P.; Sai, H.; Stern, C.L.; Stupp, S.I. Enhanced Out-of-Plane Conductivity and Photovoltaic Performance in n = 1 Layered Perovskites through Organic Cation Design. J. Am. Chem. Soc. 2018, 140, 7313–7323. [Google Scholar] [CrossRef]
- Evans, H.A.; Lehner, A.J.; Labram, J.G.; Fabini, D.H.; Barreda, O.; Smock, S.R.; Wu, G.; Chabinyc, M.L.; Seshadri, R.; Wudl, F. (TTF)Pb2I5: A Radical Cation-Stabilized Hybrid Lead Iodide with Synergistic Optoelectronic Signatures. Chem. Mater. 2016, 28, 3607–3611. [Google Scholar] [CrossRef] [Green Version]
- Maughan, A.E.; Kurzman, J.A.; Neilson, J.R. Hybrid inorganic-organic materials with an optoelectronically active aromatic cation: (C7H7)2SnI6 and C7H7PbI3. Inorg. Chem. 2015, 54, 370–378. [Google Scholar] [CrossRef]
- Van Gompel, W.T.M.; Herckens, R.; Denis, P.-H.; Mertens, M.; Gélvez-Rueda, M.C.; Van Hecke, K.; Ruttens, B.; D’Haen, J.; Grozema, F.C.; Lutsen, L.; et al. 2D layered perovskite containing functionalised benzothieno-benzothiophene molecules: Formation, degradation, optical properties and photoconductivity. J. Mater. Chem. C 2020, 8, 7181–7188. [Google Scholar] [CrossRef]
- Pious, J.K.; Basavarajappa, M.G.; Muthu, C.; Krishna, N.; Nishikubo, R.; Saeki, A.; Chakraborty, S.; Vijayakumar, C. Anisotropic Photoconductivity and Long-Lived Charge Carriers in Bismuth-Based One-Dimensional Perovskite with Type-IIa Band Alignment. J. Phys. Chem. Lett. 2020, 11, 6757–6762. [Google Scholar] [CrossRef]
- Lédée, F.; Audebert, P.; Trippé-Allard, G.; Galmiche, L.; Garrot, D.; Marrot, J.; Lauret, J.-S.; Deleporte, E.; Katan, C.; Even, J.; et al. Tetrazine molecules as an efficient electronic diversion channel in 2D organic–inorganic perovskites. Mater. Horiz. 2021, 8, 1547–1560. [Google Scholar] [CrossRef]
- Marchal, N.; Van Gompel, W.; Gélvez-Rueda, M.C.; Vandewal, K.; Van Hecke, K.; Boyen, H.-G.; Conings, B.; Herckens, R.; Maheshwari, S.; Lutsen, L.; et al. Lead-Halide Perovskites Meet Donor–Acceptor Charge-Transfer Complexes. Chem. Mater. 2019, 31, 6880–6888. [Google Scholar] [CrossRef]
- Van Gompel, W.T.M.; Herckens, R.; Van Hecke, K.; Ruttens, B.; D’Haen, J.; Lutsen, L.; Vanderzande, D. Towards 2D layered hybrid perovskites with enhanced functionality: Introducing charge-transfer complexes via self-assembly. Chem. Commun. 2019, 55, 2481–2484. [Google Scholar] [CrossRef]
- Gélvez-Rueda, M.C.; Van Gompel, W.T.M.; Herckens, R.; Lutsen, L.; Vanderzande, D.; Grozema, F.C. Inducing Charge Separation in Solid-State Two-Dimensional Hybrid Perovskites through the Incorporation of Organic Charge-Transfer Complexes. J. Phys. Chem. Lett. 2020, 11, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.-H.; You, M.-H.; Lin, M.-J. Encapsulating third donors into D–A hybrid heterostructures to form three-component charge-transfer complexes for enhanced electrical properties. Dalton Trans. 2021, 50, 13961–13967. [Google Scholar] [CrossRef] [PubMed]
- Milić, J.V. Multifunctional layered hybrid perovskites. J. Mater. Chem. C 2021, 9, 11428–11443. [Google Scholar] [CrossRef]
- Goetz, K.P.; Vermeulen, D.; Payne, M.E.; Kloc, C.; McNeil, L.E.; Jurchescu, O.D. Charge-transfer complexes: New perspectives on an old class of compounds. J. Mat. Chem. C 2014, 2, 3065–3076. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Zhang, K.K.; Long, Y.; Hu, W.; Kloc, C. Tuning of the degree of charge transfer and the electronic properties in organic binary compounds by crystal engineering: A perspective. J. Mat. Chem. C 2018, 6, 1884–1902. [Google Scholar] [CrossRef]
- Shen, D.; Chen, W.-C.; Lo, M.-F.; Lee, C.-S. Charge-transfer complexes and their applications in optoelectronic devices. Mater. Today Energy 2021, 20, 100644. [Google Scholar] [CrossRef]
- Isakovskaya, K.L.; Nikovskii, I.A.; Nelyubina, Y.V. New Low-Dimensional Perovskites Based on Lead Bromide. Russ. J. Coord. Chem. 2021, 47, 365–375. [Google Scholar] [CrossRef]
- Hashidzume, A.; Zheng, Y.; Takashima, Y.; Yamaguchi, H.; Harada, A. Macroscopic Self-Assembly Based on Molecular Recognition: Effect of Linkage between Aromatics and the Polyacrylamide Gel Scaffold, Amide versus Ester. Macromolecules 2013, 46, 1939–1947. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, X.-S.; Li, X.-R.; Cao, J.-L.; Li, Z.; Jiang, Y.-B. Folded short azapeptide for conformation switching-based fluorescence sensing. Chem. Commun. 2017, 53, 13137–13140. [Google Scholar] [CrossRef]
- Murugesan, K.; Beller, M.; Jagadeesh, R.V. Reusable Nickel Nanoparticles-Catalyzed Reductive Amination for Selective Synthesis of Primary Amines. Angew. Chem. Int. Ed. 2019, 58, 5064–5068. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, H.; Tong, J.; Berry, J.J.; Beard, M.C.; Zhu, K. Advances in two-dimensional organic–inorganic hybrid perovskites. Energy Environ. Sci. 2020, 13, 1154–1186. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Liang, K.; Wang, S. Synthesis and Characterization of [NH2C(I)NH2]2ASnI5 with A = Iodoformamidinium or Formamidinium: The Chemistry of Cyanamide and Tin(II) Iodide in Concentrated Aqueous Hydriodic Acid Solutions. Inorg. Chem. 1998, 37, 321–327. [Google Scholar] [CrossRef]
- Wong, W.P.D.; Hanna, J.V.; Grimsdale, A.C. The classification of 1D ‘perovskites’. Acta Cryst. B 2020, 77, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Fieser, L.F.; Hartwell, J.L.; Jones, J.E. 9-Anthraldehyde; 2-Ethoxy-1-Naphthaldehyde. Org. Synth. 1940, 20, 11. [Google Scholar]
- Chidirala, S.; Ulla, H.; Valaboju, A.; Kiran, M.R.; Mohanty, M.E.; Satyanarayan, M.N.; Umesh, G.; Bhanuprakash, K.; Rao, V.J. Pyrene–Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials. J. Org. Chem. 2016, 81, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Scherer, A.; Diner, C.; Tykwinski, R.R. Synthesis of 1-Bromopyrene and 1-Pyrenecarbaldehyde. Org. Synth. 2016, 93, 100–114. [Google Scholar] [CrossRef]
- Yao, Q.; Kinney, E.P.; Yang, Z. Ligand-Free Heck Reaction: Pd(OAc)2 as an Active Catalyst Revisited. J. Org. Chem. 2003, 68, 7528–7531. [Google Scholar] [CrossRef]
- Ayedi, M.A.; Le Bigot, Y.; Ammar, H.; Abid, S.; Gharbi, R.E.; Delmas, M. Synthesis of Primary Amines by One-Pot Reductive Amination of Aldehydes. Synth. Commun. 2013, 43, 2127–2133. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Jones, G.B.; Mathews, J.E. Bifunctional antitumor agents. Derivatives of pyrrolo[9,10-b]phenanthrene—A DNA intercalative delivery template. Tetrahedron 1997, 53, 14599–14614. [Google Scholar] [CrossRef]
- Alvarez, S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. [Google Scholar] [CrossRef] [Green Version]
- Eppel, S.; Fridman, N.; Frey, G. Amide-Templated Iodoplumbates: Extending Lead-Iodide Based Hybrid Semiconductors. Cryst. Growth Des. 2015, 15, 4363–4371. [Google Scholar] [CrossRef]
- Kamminga, M.E.; de Wijs, G.A.; Havenith, R.W.A.; Blake, G.R.; Palstra, T.T.M. The Role of Connectivity on Electronic Properties of Lead Iodide Perovskite-Derived Compounds. Inorg. Chem. 2017, 56, 8408–8414. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Guo, P.; Kepenekian, M.; Hadar, I.; Katan, C.; Even, J.; Schaller, R.D.; Stoumpos, C.C.; Kanatzidis, M.G. Structural Diversity in White-Light-Emitting Hybrid Lead Bromide Perovskites. J. Am. Chem. Soc. 2018, 140, 13078–13088. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Tao, X.; Li, D.-S.; Zhang, Q. Surfactants as additives make the structures of organic–inorganic hybrid bromoplumbates diverse. Inorg. Chem. Front. 2016, 3, 1388–1392. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getsis, A.; Mudring, A.V. Imidazolium based ionic liquid crystals: Structure, photophysical and thermal behaviour of C(n)mim Br center dot xH(2)O (n = 12, 14; x = 0, 1). Cryst. Res. Technol. 2008, 43, 1187–1196. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gong, C.; Gao, H.; Wen, W.; Gong, Y.; Jiang, X.; Zhang, B.; Wu, Y.; Wu, Y.; Fu, H.; et al. Single-crystalline layered metal-halide perovskite nanowires for ultrasensitive photodetectors. Nat. Electron. 2018, 1, 404–410. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, M.; Chang, B.; Sun, H.; Zheng, K.; Pullerits, T.; Chi, Q. Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics. J. Mat. Chem. C 2019, 7, 3369–3374. [Google Scholar] [CrossRef] [Green Version]
- García de Arquer, F.P.; Armin, A.; Meredith, P.; Sargent, E.H. Solution-processed semiconductors for next-generation photodetectors. Nat. Rev. Mat. 2017, 2, 16100. [Google Scholar] [CrossRef] [Green Version]







| Parameter | [AntrNH3][PbBr3](DMF) | [PyrNH3][Pb2Br6][DMFH](DMF)2 | [StyrNH3]2[PbBr4] |
|---|---|---|---|
| Formula unit | C36H42Br6N4O2Pb2 | C26H36Br6N4O3Pb2 | C30H32Br4N2Pb |
| Formula weight | 1456.57 | 1346.43 | 947.40 |
| T, K | 100 | 120 | 100 |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic |
| Space group | P212121 | P21/n | Cc |
| Z | 4 | 4 | 16 |
| a, Å | 4.3768(2) | 7.5989(7) | 22.6541(4) |
| b, Å | 20.9524(8) | 22.120(2) | 15.7611(3) |
| c, Å | 44.9467(15) | 22.258(2) | 36.9741(7) |
| β, ° | 90 | 92.060(2) | 95.2120(10) |
| V, Å3 | 4121.8(3) | 3738.9(6) | 13147.1(4) |
| Dcalc (g cm−1) | 2.347 | 2.392 | 1.915 |
| Linear absorption, μ (cm−1) | 140.12 | 154.37 | 100.16 |
| F(000) | 2704 | 2472 | 7168 |
| 2Θmax, ° | 54 | 52 | 54 |
| Reflections measured | 85071 | 36079 | 200887 |
| Independent reflections | 9012 | 7354 | 28716 |
| Observed reflections [I > 2σ (I)] | 8637 | 5245 | 25396 |
| Parameters | 456 | 376 | 1260 |
| R1 | 0.0377 | 0.0399 | 0.0500 |
| wR2 | 0.0843 | 0.0944 | 0.1341 |
| GOOF | 1.138 | 1.042 | 1.039 |
| Δρmax/Δρmin (e Å−3) | 1.620/−1.673 | 1.972/−1.598 | 3.283/−1.633 |
| Parameter | [AntrNH3][PbBr3](DMF) | [PyrNH3][Pb2Br6][DMFH](DMF)2 | [StyrNH3]2[PbBr4] |
|---|---|---|---|
| Pb-Br, Å | 2.8480(10)—3.1916(10) [2.8402(11)—3.2532(13)] | 2.9250(9)—3.1786(10) [2.8569(9)—3.3482(10)] | 2.8717(19)—3.209(2) [2.841(2)—3.225(2), 2.8587(18)—3.232(3), 2.870(2)—3.192(2)] |
| N…Br, Å | 3.289(11)—3.415(11) | 3.537(7) | 3.232(14)—3.701(13) |
| NHBr, ° | 131.2(8)—150.0(7)° | 159.4(4) | 119(1)—172(1) |
| N…O, Å | 2.767(14), 2.771(14) | 2.801(9), 2.835(9) | - |
| NHO, ° | 168.8(7), 171.3(8) | 158.5(4), 163.1(5) | - |
| O…O, Å | - | 2.42(1) | - |
| OHO, ° | - | 166.0(5) | - |
| S(Oh) | 0.122 [0.282] | 0.998 [1.138] | 0.866 [0.397, 0.885, 0.352] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikovskiy, I.A.; Isakovskaya, K.L.; Nelyubina, Y.V. New Low-Dimensional Hybrid Perovskitoids Based on Lead Bromide with Organic Cations from Charge-Transfer Complexes. Crystals 2021, 11, 1424. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111424
Nikovskiy IA, Isakovskaya KL, Nelyubina YV. New Low-Dimensional Hybrid Perovskitoids Based on Lead Bromide with Organic Cations from Charge-Transfer Complexes. Crystals. 2021; 11(11):1424. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111424
Chicago/Turabian StyleNikovskiy, Igor A., Kseniya L. Isakovskaya, and Yulia V. Nelyubina. 2021. "New Low-Dimensional Hybrid Perovskitoids Based on Lead Bromide with Organic Cations from Charge-Transfer Complexes" Crystals 11, no. 11: 1424. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11111424