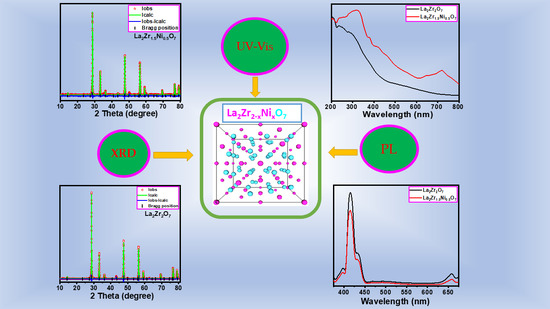
Effect of Preparation Method and Ni2+ Substitution on the Structural, Thermal, and Optical Properties of Nanocrystalline Lanthanum Zirconate Pyrochlore
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Pyrochlores (La2Zr2-xNixO7)
- (a)
- Solid-State Method
- (b)
- Sol-Gel Auto-Combustion Method
2.2. Characterization
3. Results and Discussion
3.1. Thermal Study
3.2. Crystal Structural Analysis by XRD
3.3. FT-IR Study
3.4. Textural Properties
3.5. UV–Visible Diffused Reflectance Spectroscopy Study
3.6. Photoluminescence Study
3.7. Chemical Analysis by EDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakoumakos, B.C. Systematics of the pyrochlore structure type, ideal A2B2X6Y. J. Solid State Chem. 1984, 53, 120–129. [Google Scholar] [CrossRef]
- Pirzada, M.; Grimes, R.W.; Maguire, J.; Sickafus, K. Predictions of strontium accommodation in A2B2O7 pyrochlores. J. Mater. Res. 2002, 17, 2041–2047. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, M.A.G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 5, 155. [Google Scholar] [CrossRef]
- Heremans, C.; Wuensch, B.J.; Stalick, J.K.; Prince, E. Fast-Ion Conducting Y2(ZryTi1−y)2O7 Pyrochlores: Neutron Rietveld Analysis of Disorder Induced by Zr Substitution. J. Solid State Chem. 1995, 117, 108–121. [Google Scholar] [CrossRef]
- Zhang, F.X.; Lang, M.; Tracy, C.; Ewing, R.C.; Gregg, D.J.; Lumpkin, G.R. Incorporation of uraniumin pyrochlore oxides and pressure-induced phase transitions. J. Solid State Chem. 2014, 219, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Gaura, S.; Haynesc, D.J.; Spiveya, J.J. Rh, Ni, and Ca substituted pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2011, 403, 142–151. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Shekhawat, D.; Haynes, D.J.; Spivey, J.J. The effect of La substitution by Sr- and Ca- in Ni substi-tuted Lanthanum Zirconate pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2020, 602, 117721. [Google Scholar] [CrossRef]
- Zhong, F.; Zhao, J.; Shi, L.; Cai, G.; Zheng, Y.; Zheng, Y.; Xiao, Y.; Jiang, L. Pyrochlore Pr2Zr1.95In0.05O7+δ oxygen conductors: De-fect-induced electron transport and enhanced NO2 sensing performances. Electrochim. Acta 2019, 293, 338–347. [Google Scholar] [CrossRef]
- Lee, K.-S.; Seo, D.-K.; Whangbo, M.-H. Structural and Electronic Factors Governing the Metallic and Nonmetallic Properties of the PyrochloresA2Ru2O7−y. J. Solid State Chem. 1997, 131, 405–408. [Google Scholar] [CrossRef]
- Shafique, M.; Kennedy, B.J.; Iqbal, Y.; Ubic, R. The effect of B-site substitution on structural transformation and ionic conductivity in Ho2(ZryTi1−y)2O7. J. Alloys Compd. 2016, 671, 226–233. [Google Scholar] [CrossRef]
- Oh, S.H.; Black, R.; Pomerantseva, E.; Lee, J.-H.; Nazar, L. Synthesis of a metallic mesoporous pyrochlore as a catalyst for lithium–O2 batteries. Nat. Chem. 2012, 4, 1004–1010. [Google Scholar] [CrossRef]
- Mansingh, S.; Acharya, R.; Martha, S.; Parida, K.M. Pyrochlore Ce2Zr2O7 decorated over rGO: A photocatalyst that proves to be efficient towards the reduction of 4-nitrophenol and degradation of ciprofloxacin under visible light. Phys. Chem. Chem. Phys. 2018, 20, 9872–9885. [Google Scholar] [CrossRef]
- Ajabshir, S.Z.; Salehi, Z.; Niasari, M.S. Synthesis of dysprosium cerate nanostructures using Phoenix dactylifera extract as novel green fuel and investigation of their electrochemical hydrogen storage and Coulombic efficiency. J. Clean. Prod. 2019, 215, 480–487. [Google Scholar] [CrossRef]
- Christopher, J.; Swamy, C.S. Surface characterization and catalytic activity of Ln2Ti2O7 (Ln = Y, Sm, Gd and Tb). J. Mater. Sci. 1991, 26, 4966–4970. [Google Scholar] [CrossRef]
- Gaudet, J.; Smith, E.M.; Dudemaine, J.; Beare, J.; Buhariwalla, C.R.C.; Butch, N.P.; Stone, M.B.; Kolesnikov, A.I.; Xu, G.; Yahne, D.R.; et al. Quantum Spin Ice Dynamics in the Dipole-Octupole Pyrochlore Magnet Ce2Zr2O7. Phys. Rev. Lett. 2019, 122, 187201. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Li, J.; Ma, C.; Hao, Z. Catalytic combustion of methane over La2TM0.3Zr1.7O7−δ (TM = Mn, Fe, and Co) pyrochlore oxides. Catal. Commun. 2009, 10, 1170–1173. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Panet, Y. Progresses in the Preparation of Coke Resistant Ni-based Catalyst for Steam and CO2 Reform-ing of Methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Ramachandran, C.S.; Balasubramanian, V.; Ananthapadmanabhan, P.V. Thermal cycling behavior of plasma sprayed lanthanum zirconate based coatings under concurrent infiltration by a molten glass concoction. Ceram. Int. 2013, 39, 1413–1431. [Google Scholar]
- Urban, S.; Djerdj, I.; Dolcet, P.; Chen, L.; Möller, M.; Khalid, O.; Camuka, H.; Ellinghaus, R.; Li, C.; Gross, S.; et al. In Situ Study of the Oxygen-Induced Transformation of Pyrochlore Ce2Zr2O7+x to the κ-Ce2Zr2O8 Phase. Chem. Mater. 2017, 29, 9218–9226. [Google Scholar] [CrossRef]
- Modeshia, D.R.; Walton, R.I. Solvothermal synthesis of perovskites and pyrochlores: Crystallisation of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 39, 4303–4325. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Wang, L.; Sun, X.; Yang, C.; Zou, Z.; Li, X. Hydrothermal assisted synthesis and hot-corrosion resistance of nano lanthanum zirconate particles. Ceram. Int. 2014, 40, 3981–3988. [Google Scholar] [CrossRef]
- Omata, T.; Ikeda, K.; Tokashiki, R.; Otsuka-Yao-Matsuo, S. Proton solubility for La2Zr2O7 with a pyrochlore structure doped with a series of alkaline-earth ions. Solid State Ion. 2004, 167, 389–397. [Google Scholar] [CrossRef]
- Bai, Y.; Lu, L.; Bao, J. Synthesis and Characterization of Lanthanum Zirconate Nanocrystals Doped with Iron Ions by a Salt-Assistant Combustion Method. J. Inorg. Organomet. Polym. Mater. 2011, 21, 590–594. [Google Scholar] [CrossRef]
- Joulia, A.; Vardelle, M.; Rossignol, S. Synthesis and thermal stability of Re2Zr2O7, (Re = La, Gd) and La2(Zr1−xCex)2O7−δ com-pounds under reducing and oxidant atmospheres for thermal barrier coatings. J. Eur. Ceram. Soc. 2013, 33, 2633–2644. [Google Scholar] [CrossRef]
- Tang, H.; Sun, H.; Chen, D.; Jiao, X. Fabrication and characterization of nanostructured La2Zr2O7 fibers. Mater. Lett. 2012, 70, 48–50. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Deng, S.; Wang, P.; He, Y. Direct preparation of La2Zr2O7 microspheres by cathode plasma electrolysis. J. Colloid Interface Sci. 2016, 474, 146–150. [Google Scholar] [CrossRef]
- Saradhi, M.P.; Ushakov, S.V.; Navrotsky, A. Fluorite-pyrochlore transformation in Eu2Zr2O7—Direct calorimetric measurement of phase transition, formation and surface enthalpies. RSC Adv. 2012, 2, 3328–3334. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.; Feng, X.; Li, X.; Liu, W.; Xu, X.; Zhang, N.; Gao, Z.; Wang, X.; Zhou, W. Ni/Ln2Zr2O7 (Ln = La, Pr, Sm and Y) catalysts for methane steam reforming: The effects of A site replacement. Catal. Sci. Technol. 2017, 7, 2729–2743. [Google Scholar] [CrossRef]
- Saitzek, S.; Shao, Z.; Bayart, A.; Ferri, A.; Huvé, M.; Roussel, P.; Desfeux, R. Ferroelectricity in La2Zr2O7 thin films with a frustrated pyrochlore-type structure. J. Mater. Chem. C 2014, 2, 4037–4043. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhang, L.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of perovskite LaFeO3 nanocrystals. Mater. Lett. 2006, 60, 1767–1770. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, J.; Yue, Z.; Gui, Z.; Li, L. Auto-combustion synthesis of nanocrystalline LaFeO3. Mater. Chem. Phys. 2003, 78, 25–29. [Google Scholar] [CrossRef]
- Carbonin, S.; Martignago, F.; Menegazzo, G.; Negro, A. X-ray single-crystal study of spinels: In situ heating. Phys. Chem. Miner. 2002, 29, 503–514. [Google Scholar] [CrossRef]
- Kumar, L.; Kumar, P.; Narayan, A.; Kar, M. Rietveld analysis of XRD patterns of different sizes of nanocrystalline cobalt ferrite. Int. Nano Lett. 2013, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Kar, M. Effect of La3+ substitution on the structural and magnetocrystalline anisotropy of nanocrystalline cobalt ferrite (CoFe2-xLaxO4). Ceram. Int. 2012, 38, 4771–4782. [Google Scholar] [CrossRef]
- Hatnean, M.C.; Lees, M.; Balakrishnan, G. Growth of single-crystals of rare-earth zirconate pyrochlores, Ln2Zr2O7 (with Ln = La, Nd, Sm, and Gd) by the floating zone technique. J. Cryst. Growth 2015, 418, 1–6. [Google Scholar] [CrossRef]
- Culity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Series; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Suryanarayana, C.; Grant Nortan, M. X-ray Diffraction: A Practical Approach; Plenum Publishing Corporation: New York, NY, USA, 1998. [Google Scholar]
- Tangwiwat, S.; Milne, S.J. Barium titanate sols prepared by a diol-based sol–gel route. J. Non-Cryst. Solids 2005, 351, 976–980. [Google Scholar] [CrossRef]
- Slamovich, E.B.; Aksay, I.A. Structure Evolution in Hydrothermally Processed (<100 °C) BaTiO3 Films. J. Am. Ceram. Soc. 1996, 79, 239. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.A.; Arunachalam, P.; Madhavan, J.; Ghanem, M.A. Synthesis of Ni3V2O8@graphene oxide nanocomposite as an efficient electrode material for supercapacitor applications. J. Solid State Electrochem. 2018, 22, 527–536. [Google Scholar] [CrossRef]
- Kambale, R.C.; Song, K.M.; Koo, Y.S.; Hur, N. Low temperature synthesis of nanocrystalline Dy3+ doped cobalt ferrite: Structural and magnetic properties. J. Appl. Phys. 2011, 110, 053910. [Google Scholar] [CrossRef]
- Dos Santos, M.; Lima, R.; Riccardi, C.; Tranquilin, R.; Bueno, P.; Varela, J.; Longo, E. Preparation and characterization of ceria nanospheres by microwave-hydrothermal method. Mater. Lett. 2008, 62, 4509–4511. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, T.; Li, C.; Ma, J. Peapod-like nickel@mesoporous carbon core-shell nanowires: A novel electrode material for supercapacitors. RSC Adv. 2011, 1, 954–957. [Google Scholar] [CrossRef]
- Jayaraman, V.; Mani, A. Interfacial coupling effect of high surface area Pyrochlore like Ce2Zr2O7 over 2D g-C3N4 sheet pho-toactive material for efficient removal of organic Pollutants. Sep. Purif. Technol. 2020, 235, 116242. [Google Scholar] [CrossRef]
- Bencina, M.; Valant, M. Bi2Ti2O7-based pyrochlore nanoparticles and their superior photocatalytic activity under visible light. J. Am. Ceram. Soc. 2018, 101, 82–90. [Google Scholar] [CrossRef]
- Gan, Y.P.; Qin, H.P.; Huang, H.; Tao, X.Y.; Fang, J.W.; Zhang, W.K. Preparation and Photocatalytic Activity of Rutile TiO2-Graphene Composites. Acta Phys. Chim. Sin. 2013, 29, 403–410. [Google Scholar]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based compo-sites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, P.Y.; Khataee, A.; Sadeghi Rad, T.; Hassani, A.; Joo, S.W. Fabrication of ZnFe-layered double hydroxides with graphene oxide for efficient visible light photocatalytic performance. J. Taiwan Inst. Chem. Eng. 2019, 101, 186–203. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Mangalaraja, R.V.; Sathishkumar, P.; Murugesan, S.; Muneeswaran, T.; Pandiyarajan, T.; Naveenraj, S.; Contreras, D.; Anandan, S. Green synthesis of porous Au–Nx-TiO2 nanospheres for solar light induced photocatalytic degradation of diazo and triazo dyes and their eco-toxic effects. New J. Chem. 2018, 42, 18717–18728. [Google Scholar] [CrossRef]











| Sample | Volume (Å)3 | Unit Cell (Å) | Crystallite Size (nm) | R-Factors | GOF | |||
|---|---|---|---|---|---|---|---|---|
| a = b = c | S. Method | W–H Method | Rp | Rexp | Rwp | χ2 | ||
| LZ-sg | 1271.97 | 10.8349 ± 0.0001 | 40.80 | 35.15 | 13.18 | 13.40 | 16.27 | 1.47 |
| LZN-sg | 1271.73 | 10.8342 ± 0.0001 | 63.00 | 59.36 | 12.05 | 13.86 | 15.35 | 1.21 |
| Sample | Atoms | Site | x | y | z | Occupancy |
|---|---|---|---|---|---|---|
| LZ-sg | ||||||
| La | 16c | 0 | 0 | 0 | 1 | |
| Zr | 16d | 0.5 | 0.5 | 0.5 | 1 | |
| O1 | 48f | 0.42 | 0.125 | 0.125 | 1 | |
| O2 | 8a | 0.125 | 0.125 | 0.125 | 1 | |
| LZN-sg | ||||||
| La | 16c | 0 | 0 | 0 | 1 | |
| Zr/Ni | 16d | 0.5 | 0.5 | 0.5 | 0.9430 | |
| O1 | 48f | 0.42 | 0.125 | 0.125 | 0.9991 | |
| O2 | 8a | 0.125 | 0.125 | 0.125 | 0.9933 |
| Sample | SBET (cm2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| LZ-sg | 2.35 | 0.0074 | 21.11 |
| LZN-sg | 2.05 | 0.0079 | 24.02 |
| Sample | Excitation at 350 nm | Excitation at 400 nm | BE | BE a | |||
|---|---|---|---|---|---|---|---|
| 350 nm | 400 nm | ||||||
| LZ-sg | 415 | 657 | 450 | 635 | 2.98 | 2.7 | 2.9 |
| LZN-sg | 415 | 657 | 450 | 635 | 2.98 | 2.7 | 3.2 |
| Sample | Weight% | Atomic% | ||||||
|---|---|---|---|---|---|---|---|---|
| La | Zr | Ni | O | La | Zr | Ni | O | |
| LZ-sg | 34.98 | 34.43 | 25.80 | 9.54 | 14.29 | 61.06 | ||
| LNZ-sg | 39.85 | 27.14 | 5.14 | 24.06 | 11.50 | 11.93 | 3.51 | 60.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, F.; Wahab, R.; Manoharadas, S.; Alrayes, B.F.; Ahmad, N. Effect of Preparation Method and Ni2+ Substitution on the Structural, Thermal, and Optical Properties of Nanocrystalline Lanthanum Zirconate Pyrochlore. Crystals 2021, 11, 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121463
Alharthi F, Wahab R, Manoharadas S, Alrayes BF, Ahmad N. Effect of Preparation Method and Ni2+ Substitution on the Structural, Thermal, and Optical Properties of Nanocrystalline Lanthanum Zirconate Pyrochlore. Crystals. 2021; 11(12):1463. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121463
Chicago/Turabian StyleAlharthi, Fahad, Rizwan Wahab, Salim Manoharadas, Basel F. Alrayes, and Naushad Ahmad. 2021. "Effect of Preparation Method and Ni2+ Substitution on the Structural, Thermal, and Optical Properties of Nanocrystalline Lanthanum Zirconate Pyrochlore" Crystals 11, no. 12: 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121463